Research outcomes of the Program for the Promotion of Next Generation Research Projects
Yoko Ozawa
School of Medicine
Subproject 1: Retinal degeneration and mitochondrial dysfunction
Mitochondrial dysfunction can cause many neural degenerative diseases, including age-related macular degeneration. To investigate the mitochondria-related mechanism of retinal degeneration, we utilized induced pluripotent stem cells (iPSCs) derived from a patient with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS), who has a mutation of tRNA gene in the mitochondrial DNA (mtDNA). MELAS iPSCs showed lower oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) than those in control iPSCs. In concordance with the data, gene expression of respiratory enzymes, such as the beta subunit of lactate dehydrogenase enzyme (LDHB), pyruvate dehydrogenase E1 subunit alpha 1 (PDHA1), and pyruvate dehydrogenase kinase 1 (PDK1), a mitochondrial genome coding gene, NADH-ubiquinone oxidoreductase core subunit 6 (ND6), a glycolysis related enzyme, hexokinase-2 (HK2), and glucose transporters -1 (SLC2A1, GLUT1) and -3 (SLC2A3, GLUT3), were downregulated in MELAS iPSCs. The drug screening system by the live/dead cell imaging indicated MELAS iPSCs were vulnerable to glucose deficiency, which was partly rescued by taurine treatment. Metabolomic analyses showed that the ratio of the reduced (GSH) to oxidized glutathione (GSSG) was decreased whereas the levels of cysteine, a substrate of GSH, and oxidative stress markers were upregulated in MELAS iPSCs. Taurine normalized these changes, suggesting that MELAS iPSCs were affected by the oxidative stress and taurine reduced its influence. Because MELAS patients are reported to involve degeneration of the retinal pigment epithelium (RPE), RPE were differentiated by retinal organoid culture from control and MELAS iPSCs. Both control and MELAS iPSC-derived RPE (MELAS RPE) expressed an RPE marker, Otx2, and a tight junction marker, ZO-1, although the regular hexagonal cell shapes were seldom in MELAS RPE. In MELAS RPE, OCR and ECAR were decreased as compared with those in control RPE. It is known that Epithelial-Mesenchymal Transition (EMT) of the RPE is involved in the retinal diseases, such as age-related macular degeneration (AMD). We found that mRNA levels of EMT-related transcription factors, ZEB1, SNAI1 (Snail), and TWIST1, and ACTA2 (aSMA), were increased in the MELAS RPE cells compared with those in control RPE cells. Taurine treatment decreased these EMT-related transcription factors and EMT inducers, TGFB1 and TGFB2. Overall, we identified taurine as a potent drug for the treatment of mitochondria disease by MELAS iPSC-based screening system, and in MELAS RPE, EMT signals were decreased through the suppression of oxidative stress by taurine treatment (Homma K, Ozawa Y et al. Redox Biology. 2021) (Fig. 1).
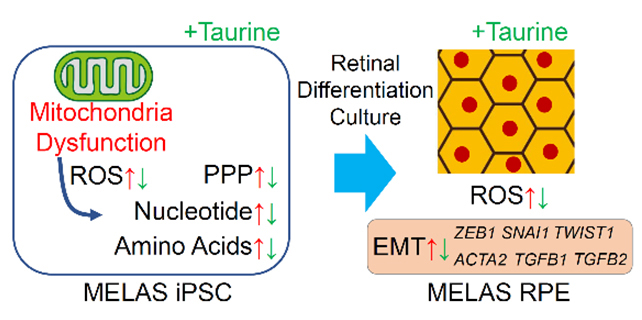
Fig. 1
Taurine reduced oxidative stress in MELAS iPSCs and EMT signals in MELAS RPE. (Left) Metabolomic analysis revealed the changes in MELAS iPSCs those were normalized by taurine treatment. (right) Taurine treatment reduced oxidative stress and EMT signals in MELAS RPE.
Subproject 2: Establishment of a cell line with homoplasmic mtDNA mutation
Mitochondrial (mt) DNA is an important factor to regulate mitochondrial functions. However, it is difficult to carry out functional analysis on mtDNA because cells each contain several thousand copies of mtDNA, and dysfunctions of the mutated mtDNA are compensated by other mtDNAs existing in the same cell. Therefore, introducing the same mutation(s) to all copies of mtDNA (i.e. achievement of homoplasmy of mutated mtDNA) is required for functional analysis of mtDNA. Single mitochondrion transfer from a mtDNA mutation-accumulated cell to a mtDNA-less (ρ0) cell is a promising approach to achieve homoplasmy of mutated mtDNA; however, a practical method for this is not yet available. The purpose of this study was to develop a method for single mitochondrion transfer between live cells.
We previously developed a microfluidic device in which paired single cells were fused through a microslit to promote a strictured cytoplasmic connection. In this situation, mitochondria gradually migrated to the fusion partner passing through the cytoplasmic connection. Based on this result, we hypothesized that elongating the length of the strictured cytoplasmic connection would result in a smaller amount of mitochondrial transfer because of the difficulty in passing through the cytoplasmic connection. To test this hypothesis, microfluidic devices with a microtunnel length of 4.1 μm, 5.6 μm or 10.0 μm were fabricated.
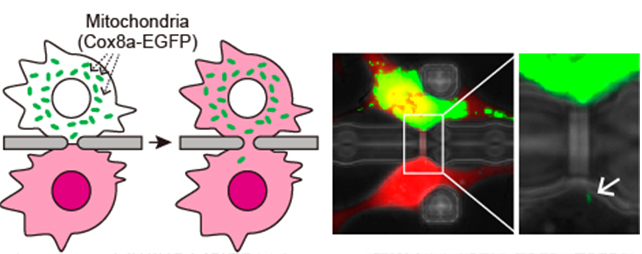
Fig. 2.
Mitochondrial transfer using a microfluidic device. (Left) Schematic illustration of mitochondrial transfer. (right) An example of migration of single mitochondrion to the fusion partner. (Wada et.al., RIKEN)
When NIH-3T3 cells were fused through a microtunnel using the Sendai virus envelope-based method, a strictured cytoplasmic connection with an analogous length to the microtunnel was successfully facilitated, and as expected, a longer cytoplasmic connection resulted in a smaller amount of mitochondria being transferred. Notably, some cell pairs fused through a 10.0 μm-length microtunnel showed migration of a single mitochondrion to the fusion partner (Fig. 2). Added to this, we found that a recovery culture using a normal growth medium disconnected the fused cells to recover into single cells. These results suggest that the cell fusion using the microfluidic device has the potential to perform single mitochondrion transfer between live cells. Finally, we confirmed mtDNA repopulation in ρ0 cells by mitochondrial transfer from mtDNA-intact cells using the microfluidic device. With the newly-obtained data, we concluded that the cell fusion method developed in this study will provide a unique mitochondrial transfer platform for the establishment of a cell line with homoplasmic mtDNA mutations (Fig. 3).
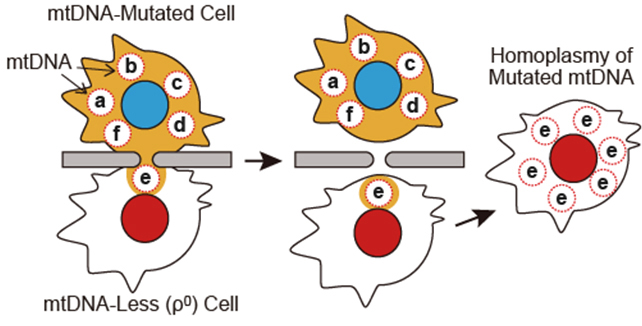
Fig. 3.
Establishment of cell line with homoplasmic mtDNA mutation using microfluidic device. Single mitochondrion transfer to ρ0 cell and subsequent mtDNA repopulation would achieve homoplasmy of mutated mtDNA. In this scheme, mitochondria are omitted.
Subproject 3: Live imaging of mitochondrial function and Mg2+ dynamics
For the purpose of investigating the intracellular function of intracellular Mg ions, we proceeded with the development of a new probe for imaging Mg ions and the visualization research of mitochondrial function using the probe. In the visualization of Mg ions by the conventional fluorescence imaging method, it was difficult to obtain multiple intracellular information at the same time due to the overlapping of the fluorescence wavelengths. Therefore, in the case of simultaneous fluorescence imaging of the interaction between Mg ions and Ca ions, a red fluorescence sensor such as Fura-red was used as a Ca ion probe. If a red Mg ion probe could be made, the possibility of performing multicolor imaging is expected to improve dramatically.
We thus developed a new Mg ion probe KMG-500 with near-infrared fluorescence (Murata et al. Analytic Chemistry, 2020) (Fig. 4). This new probe has a charged β-diketone as a selective Mg ion binding site and a photoinduced electron transfer (PeT)-type OFF-ON type sensor with a Si-rhodamine group as a near infrared dye at 650 to 750 nm. The change in the near-infrared fluorescence wavelength is caused by the binding with Mg ions. When a probe modified with an acetoxymethyl group so that the cell membrane was permeable to this type of novel probe was introduced into rat hippocampal neurons, the intracellular Mg ion concentration was successfully visualized. Since this probe shows a response in the near-infrared, ATeam, which is an ATP probe for FRET by fluorescent protein (fluorescence measurement at 450 nm and 530 nm is required), TMRE dye and a new probe can be used simultaneously (simultaneous measurement of mitochondrial membrane potential, cytoplasmic ATP concentration, and Mg ion concentration was performed by using 600 nm). By applying this triple imaging technique to rat hippocampal neurons, the response by FCCP application that depolarizes the inner mitochondrial membrane potential and the depolarization-induced release of Mg ions from mitochondria to the cytoplasm were examined. Furthermore, we were able to trace the decrease in ATP concentration due to the inhibition of mitochondrial function over time. This novel Mg ion probe is expected to accelerate studies that have previously been unclear on the function of intracellular Mg ions.
In addition to the above research, we have summarized the relationship between the role of Mg ions in nerve cells and neurological disorders such as Parkinson's disease and published it as a review article (Yamanaka, Shindo, Oka, International Journal of Molecular Sciences, 2019).
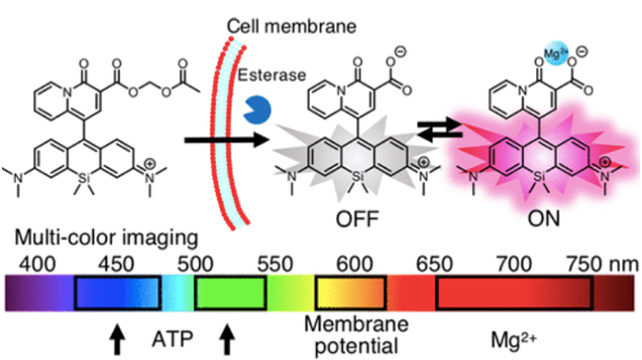
Fig. 4.
Establishment of membrane permeable red Mg2+
probe. Multiple bio imaging can be applied by using the novel red Mg2+ probe (KMG-500), the mitochondria membrane potential (TMRE), and the ATP probe (ATeam).
Keio University Program for the Promotion of Next Generation Research Projects
The Keio University Program for the Promotion of Next Generation Research Projects subsidizes research costs with the aim of finding solutions to challenges and of promoting global academic research in order to allow Keio University faculty members to establish a presence as core researchers.