Research outcomes of the Program for the Promotion of Next Generation Research Projects
Faculty of Science and Engineering
Yoshiaki Furukawa
According to the "Central Dogma" of molecular biology, the information indispensable to life encoded in DNA is first transcribed into RNA and then translated into proteins. These proteins subsequently perform various physiological reactions with extraordinary specificity and are thus central to all living organisms. For proteins to become functional, however, there is one missing but very important element--a metal ion. Believe or not, almost 30% of all proteins require metal ions for their physiological functions. The most famous example showing the importance of metal ions is hemoglobin in the red blood cells, which carries oxygen around the body. Hemoglobin binds an iron-containing compound called heme, in which the iron ion is responsible for capturing oxygen molecules. If we do not get enough iron in our diet, the resultant deficiency leads to the loss of functional hemoglobin causing anemia. Also, metal-binding proteins called "metalloproteins" can easily perform chemical reactions that are quite difficult with organic synthesis under mild conditions; for example, a copper-containing methane monooxygenase produces methanol from methane, and a molybdenum-containing nitrogenase can convert nitrogen into ammonia.
Metalloproteins are so fundamental to life and diverse in their functions that research on the metal-protein interactions should be interdisciplinary. In other words, collaborations among researchers from different fields are required to understand the roles and functions of metalloproteins. That is why, in the Program for the Promotion of Next Generation Research Projects, researchers who have an interest in metals in proteins were assembled from different faculties and departments. As an example of their collaborative achievements, the following is a brief introduction of the study on the pathological roles of a metalloprotein SOD1 (Fig. 1A) in the neurodegenerative disease ALS.
Patients with ALS suffer from a progressive loss of the ability to move and even breathe due to the selective loss of motor neurons, and unfortunately, there is currently no known cure (Fig. 1B). In a small percentage of ALS cases, mutations in the gene coding SOD1 have been detected, and mutant SOD1 proteins are known to accumulate by assuming abnormal and possibly cytotoxic conformations in degenerating motor neurons (Fig. 1C). Based upon biophysical studies with purified proteins in test tubes, Prof. Yoshiaki Furukawa of the Keio University Faculty of Science and Engineering has proposed that such abnormal or so-called "misfolded" conformations of mutant SOD1 are caused by a failure to obtain the copper and zinc ions that SOD1 would normally bind (Fig. 2)1.
In order to understand how this devastating disease occurs, Prof. Furukawa believes that this proposed mechanism in vitro should be further tested in vivo, and indeed, collaborations have made a significant contribution to solving this interdisciplinary issue. Prof. Hidemi Misawa of the Keio University Faculty of Pharmacy, a member of this project, studied the pathomechanism of ALS using model mice (Fig. 1B), and with his expertise on the handling and analysis of mouse tissue samples, they were successful in demonstrating that metal-deficient, abnormal SOD1 proteins do accumulate in spinal motor neurons of the ALS model mice2. This collaborative study suggests that ALS might be mitigated by supplying the correct metal ions to SOD1.
Misfolded SOD1 was successfully reproduced in vitro by controlling its metal-binding status, which means that the research team is now equipped with a molecular probe to monitor disease-related phenomena occurring in vivo. One of the testable phenomena was the clearance of misfolded proteins from the brain and spinal cord, and Prof. Misawa hypothesized that misfolded SOD1 could be cleared with "glymphatic flow," a recently proposed drainage for extracellular proteins in the brain and spinal cord (Fig. 3A). The flow is also known to be regulated by a water channel protein, aquaporin-4 (AQP4) (Fig. 3A), which is being pursued by another member of the project, Prof. Masato Yasui of the Keio University School of Medicine3. This resulted in a collaboration to test the roles of glymphatic flow in the ALS pathogenesis, and the misfolded SOD1 proteins in vitro were injected into several model mice and monitored (Fig. 3B). As a result of this, the clearance of the injected proteins was found to be less effective when the mice lacked AQP4 (Fig. 3C)4. The glymphatic flow is hence key to clearing misfolded and toxic SOD1 proteins from the brain and spinal cord, which might be another way to mitigate the progression of ALS.
The Program for the Promotion of Next Generation Research Projects was not limited to SOD1/ALS. - Dr. Norifumi Kawakami and Prof. Nobuhide Doi of the Department of Biosciences & Informatics at Keio University deal with the more applied aspects of metal ions in biological systems. Dr. Kawakami is testing his challenging idea that organisms can evolve so as to utilize alternative metal ions, while Prof. Doi has attempted to develop a sensor for specific metal ions by using a metal-responsive transcriptional factor5. The members of this program have expertise on various research fields ranging from molecules to cells to model animals. Their collaborations are therefore increasing the chance that new research themes will emerge on metals in biology and medicine.
As a part of the activities in this program, two international symposiums were held. The first, "Metals in ALS," was held on Keio University's Yagami Campus, with three Japanese professors and one Australian professor presenting up-to-date research on the pathological roles of metal ions in neurodegenerative diseases. The second symposium, "Keio Symposium on Bio-metals," was held at Keio's Shiba-Kyoritsu Campus and included lectures by two professors from the United States on the physiological roles of metal ions. Members in this project were also able to discuss their own research with those invited professors, which may lead to future international collaborations on metal-related research.
This three-year program has come to an end after many collaborative research activities on metal-protein interactions; nevertheless, the participants feel that the relationships formed along the way will continue to go from strength-to-strength, leading to ever more interdisciplinary and fruitful research.
1 Furukawa, Y. Chem Lett, 2021, 50, 331-341.
2 Tokuda, E. et al. Mol Neurodegener, 2017, 12, 2.
3 Vandebroek, A.A. and Yasui, M. Int J Mol Sci, 2020, 26, 1603
4 Hirose, M. et al. Neurosci Res, 2021, in press
5 Aye, S.L. et al. J Biochem, 2018 164, 341-348.
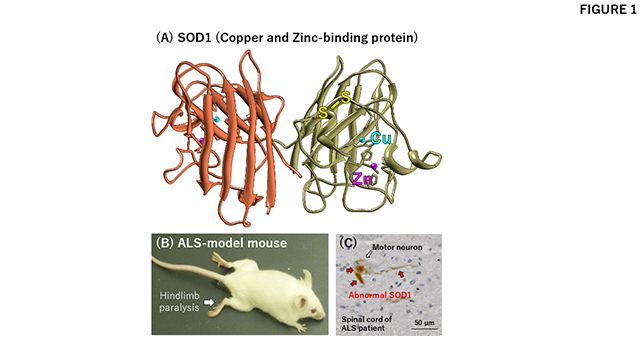
Fig. 1
(A) A three-dimensional structure of SOD1. SOD1 binds a copper (cyan) and a zinc (magenta) ion and also forms an intramolecular disulfide bond (yellow).
(B) An ALS-model mouse expressing mutant SOD1 proteins shows ALS-like symptoms including hindlimb paralysis.
(C) Spinal cord section of an ALS patient with SOD1 mutation. Abnormal (misfolded) SOD1 proteins (colored brown) accumulate in a motor neuron.
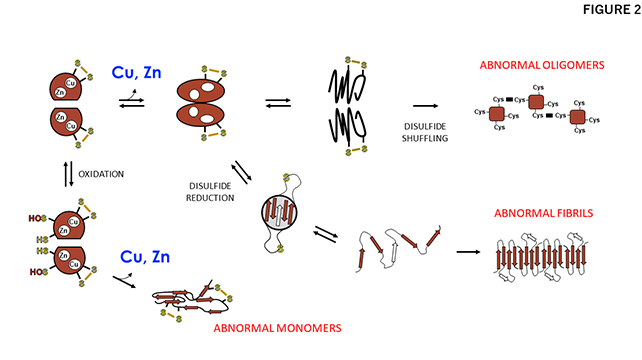
Fig. 2
A proposed mechanism for misfolding of SOD1 triggered by dissociation of the bound metal ions. Metal dissociation and/or failure to obtain metal ions decrease the structural stability of SOD1, leading to the misfolding of SOD1 into abnormal oligomers, fibrils, and monomers. As shown, the status of the disulfide bond in SOD1 is also an important factor in the misfolding.
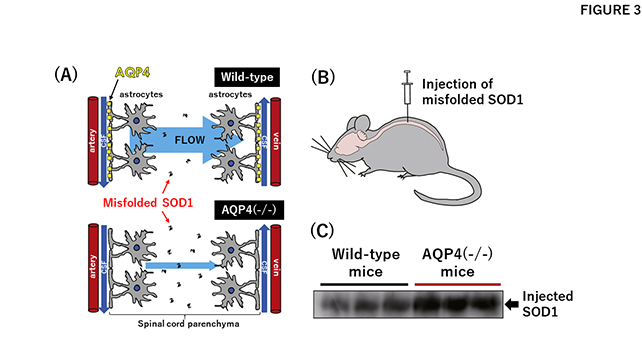
Fig. 3
Glymphatic flow plays critical roles in the clearance of misfolded SOD1 from the spinal cord.
(A) Misfolded proteins in the spinal cord parenchyma are cleared by the "flow," which is considered to be generated by the action of a water channel AQP4 (upper panel). Absence of AQP4 thus stagnates the flow, resulting in accumulation of the misfolded SOD1 in the spinal cord (lower panel).
(B) Misfolded SOD1 prepared in vitro injected into the spinal cord of a model mouse.
(C) After a certain period of time, the injected SOD1 that remained in the spinal cord tissue was quantitated. More amounts of injected SOD1 remained in the mice lacking AQP4 (AQP4(-/-)) than those of wild-type mice, supporting roles of AQP4 in clearance of the misfolded proteins.
Keio University Program for the Promotion of Next Generation Research Projects
The Keio University Program for the Promotion of Next Generation Research Projects subsidizes research costs with the aim of finding solutions to challenges and of promoting global academic research in order to allow Keio University faculty members to establish a presence as core researchers.