Research outcomes of the Program for the Promotion of Next Generation Research Projects
Hiroaki Onoe
Faculty of Science and Technology
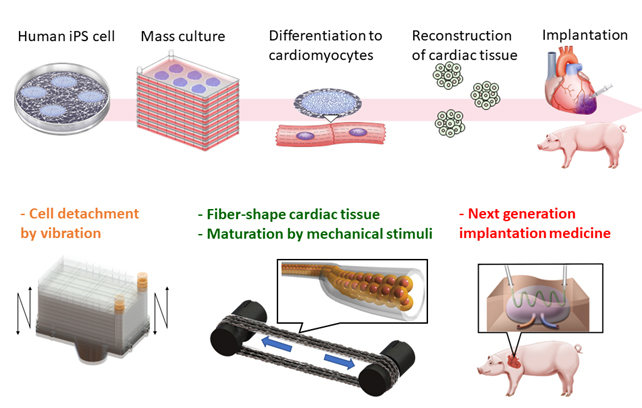
Regenerative cardiac therapies using human induced pluripotent stem (iPS) cells is an emerging treatment for severe heart failure to replace cardiac transplantation, which is hampered by a serious donor shortage problem. A group at the Department of Cardiology at the Keio University School of Medicine ― a research group that is part of our team ― has already confirmed the efficacy of human iPS-derived cardiomyocyte transplantation in small and large animals and is moving forward to conduct clinical trials for this treatment. The critical technical issues that must be overcome are the difficulty of producing a large number of high-quality cells for stable clinical trials and the organizing and maturing of cardiac tissue in the appropriate shape. Internationally, myocardial regeneration is a major target of regenerative medicine, and in vitro myocardial tissue construction has been reported from basic research to the clinical stage. However, there are three technical/cost-related issues that must be addressed:
(i) efficient mass cultivation of cells while maintaining the undifferentiated potential of iPS cells.
(ii) 3D organization and maturation of iPS cell-derived reconstructed cardiac tissue.
(iii) application of matured myocardial tissue in drug testing and regenerative medicine.
We are aiming to solve these issues through an engineering approach by controlling the mechanical factors in the culture environment. In recent years, it has become apparent that mechanical stimulation plays an important role in stem cell cultivation, differentiation, tissue formation, and maturation, and much attention has been paid to the mechanisms of these processes. Particularly for the heart and myocardium, whose function involve motion and force, the integration of tissue culture technology and tissue regenerative medicine technology that take mechanics into account may be the most appropriate method to improve efficiency and reduce costs in cultivation, tissue formation, and maturation. In other words, it would become possible to deploy iPS cell-derived cardiomyocytes in clinical grade by creating a comprehensive new technology that integrates both medical and engineering perspectives, from securing cell sources to tissue formation and medical application processes.
In this research project, we addressed the following research topics with close collaboration among three research groups in the Department of Mechanical Engineering and the cardiovascular regeneration group at the School of Medicine.
Various mechanical stimuli in tissue culture environments promote human iPSC-derived cardiac tissue reconstruction and its application toward cardiac regenerative medicine © Keio University
Research topic 1: High-efficiency mass cultivation of iPS cells using mechanical vibration-induced cell detachment
To improve the efficiency of iPS cell cultivation, the Takemura and cardiovascular regeneration groups developed a novel method to grow cultures that automates the detachment process, which until now was usually done manually. In this method, the multilayered culture plates (five layers) used for mass cultivation of human iPS cells are forced to vibrate, and each culture layer resonates in out-of-plane direction to detach the cells with high efficiency. They confirmed that this process improved the cell recovery rate and preserved the undifferentiated capacity of the cells. They also developed a prototype detachment device and confirmed that the iPS cells maintained their functions after long-term cultivation (10 passages) using this device. The results of the cell detachment method through vibration were published in four international journals (Biotechnology and Bioprocess Engineering, June 2019; Engineering in Life Science, August 2019; Communications Biology, October 2019; Scientific Reports, October 2019).
Research topic 2: Stabilization of microfiber-shaped iPS cell-derived myocardial tissue
The Onoe group constructed microfiber-shaped hydrogel-embedded cardiac tissue using human iPS cell-derived cardiomyocytes that had been highly purified by the cardiovascular regeneration group. The microfiber-shaped cardiac tissues were cultured for 2 to 4 weeks for maturation. To stabilize the tissue, fibroblasts were mixed into the human iPS cell-derived myocardium at various ratios, as well as vascular endothelial cells to support tissue formation. They found that the addition of a different type of cell was effective in stabilizing the cardiac tissue. Furthermore, the removal of the calcium alginate gel covering the microfiber-shaped tissue enabled them to assemble macroscopic tissue. A paper on this method for constructing co-cultured tissue in fiber-like tissue was published in, and featured on the cover, of the November 2019 issue of APL Bioengineering.
Research topic 3: Maturation of myocardial tissue in vitro through mechanical stimulation
The Miyata group has developed a device for maturing three-dimensional in vitro myocardial tissue by applying mechanical stimulation to fiber-shaped tissue that was fabricated in collaboration with the Onoe group. They successfully cultured fiber-shaped three-dimensional skeletal muscle tissue (C2C12) by grasping and fixing the tissue in a culture vessel and applying a tensile deformation of 0.3 to 3% of the total length at 1 Hz for about 7 days, and observed that stimulation enhanced cell proliferation and maturation of the muscle fiber tissue. They succeeded in establishing a technique for applying continuous mechanical stimulation to a fiber-shaped three-dimensional tissue. To apply their system to the regeneration of cardiac muscle, a fiber-shaped 3D tissue (cardiomyocyte cell line HL-1) was cultivated by applying a tensile deformation of 30% of its total length at 1 Hz. The results showed that mechanical stimulation enhanced the multinucleation of cells and the expression of MHC, one of the markers for assessing the maturity of myocardial tissue. The results related to this research were published in the open-access international journal Micromachines in the June 2019 issue.
Reference
- Takumi Inui, Yuta Kurashina, Chikahiro Imashiro and Kenjiro Takemura, Method of localized removal of cells using a bolt-clamped Langevin transducer with an ultrasonic horn, Engineering in Life Sciences, Volume19, Issue 8, 575-583 (2019) | article
- Yuta Kurashina, Chikahiro Imashiro, Makoto Hirano, Taiki Kuribara, Kiichiro Totani, Kiyoshi Ohnuma, James Friend and Kenjiro Takemura, Enzyme-free release of adhered cells from standard culture dishes using intermittent ultrasonic traveling waves, Communications Biology, 2, 393 (2019) | article
- Yusuke Terao, Yuta Kurashina, Shugo Tohyama, Yuki Fukuma, Keiichi Fukuda, Jun Fujita and Kenjiro Takemura, An effective detachment system for human induced pluripotent stem cells cultured on multilayered cultivation substrates using resonance vibrations, Scientific Reports 9, 15655 (2019) | article
- Yuta Kurashina, Ryo Sato, and Hiroaki Onoe, Microfiber-shaped hepatic tissues with endothelial networks for constructing macroscopic tissue assembly, APL Bioengineering 3, 046101 (2019) | article
- Shinako Bansai, Takashi Morikura, Hiroaki Onoe and Shogo Miyata, Effect of Cyclic Stretch on Tissue Maturation in Myoblast-Laden Hydrogel Fibers, Micromachines 2019, 10, 399. | article
Keio University Program for the Promotion of Next Generation Research Projects
The Keio University Program for the Promotion of Next Generation Research Projects subsidizes research costs with the aim of finding solutions to challenges and of promoting global academic research in order to allow Keio University faculty members to establish a presence as core researchers.