Research outcomes of the Program for the Advancement of Next Generation Research Projects
School of Medicine
Takeyuki Kono
Recurrent respiratory papillomatosis (RRP) arising in the mucosal epithelium of the head and neck region is caused by low-risk human papillomavirus (HPV) types 6 and 11. However, the cellular and molecular mechanisms underlying its pathogenesis remain to be elucidated. Recently, the conditional reprogramming (CR) method, which reprograms epithelial cells to an undifferentiated stem cell state, has been shown to be effective for creating preclinical models of RRP. Here, we investigated the relationship between viral and host gene expression in cells isolated by the CR method and explored the molecular pathogenesis of RRP.
When the CR method was applied, using the combination of a J2 fibroblast feeder and Rho kinase (ROCK) inhibitor, the isolated undifferentiated epithelial cells had a large nuclear ratio and a dense, cobblestone-like morphology (Figure 1A). When the ROCK inhibitor was removed from the medium, the cells stopped growing. In contrast, when the J2 feeder was not added, the cells grew, but the epithelial cells started differentiation and could no longer be passaged (Figure 1B). This suggests that both the J2 feeder and the ROCK inhibitor are essential to maintain the epithelial cells in an undifferentiated and proliferative state. There were no obvious differences in morphology and growth speed between the normal cells and tumor cells.
We attempted to establish primary cells from 14 patients and obtained 12 RRP cells and 13 normal epithelial cells from the same individuals, given that the efficiency of cell establishment was almost 90%. Established cells can be passively cultured and repopulated after freezing and thawing. The presence of HPV genomes in these cells was confirmed through PCR, RNA in situ hybridization (RNA-ISH; Figure 1C), and RNA sequencing (RNA-seq), identifying HPV6 in 10 cases and HPV11 in 2 cases. Importantly, the viral genome was maintained in the cultured cells even after multiple passages. Furthermore, we generated three-dimensional (3D) cultures by embedding these primary cells in atelocollagen gels. The morphology of RRP 3D culture cells showed a rough surface, whereas the 3D cells from normal epithelial cells had a smooth surface (Figure 2A). Frozen sections from these 3D cultures revealed expression of the HPV viral protein E4, the epithelial marker cytokeratin 5 (CK5), and the proliferation marker Ki67, indicating that the tumor characteristics were recapitulated in vitro (Figure 2B).
Using these established cell models, we analyzed changes in the expression of immune-related factors in RRP cells via PCR, immunostaining, and Western blotting. Normal mucosal epithelial cells from non-tumorous regions of the same individuals were used as a control. We particularly focused on the cGAS-STING pathway, which is a critical part of the innate immune system that detects cytosolic DNA, typically within viruses, bacteria, or damaged host cells. This was due to previous studies having shown that high-risk type HPV manipulates cGAS-STING pathways to evade host immune surveillance. Compared to normal cells, papilloma-derived cells exhibited suppressed expression of components of the cGAS-STING pathway (e.g., cGAS and IRF3), resulting in limited production of interferons. Consequently, PD-L1 expression, which is regulated via the JAK-STAT pathway, was also found to be downregulated (Figure 3). RNA-seq analysis revealed that neutrophil chemotactic genes such as CXCL1, CXCL2, and CXCL8 exhibited expression patterns similar to those of HPV genes, suggesting a potential role of HPV in regulating the expression of neutrophil-attracting chemokines.
Collectively, these findings elucidate part of the mechanism by which low-risk HPV evades host immune responses in RRP and provide new insights into potential therapeutic targets for this disease.
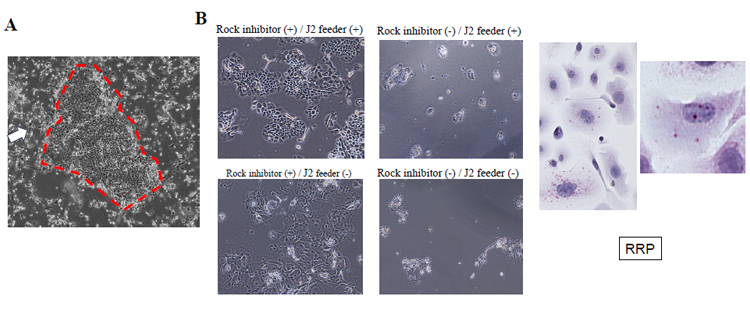
Figure 1:
Morphology of the isolated 2D cells using the conditional reprogramming method
(A) The isolated epithelial cells had a large nuclear ratio and a dense, cobblestone-like morphology (red circle). J2 fibroblast feeder cells are in the surrounding area (white arrow).
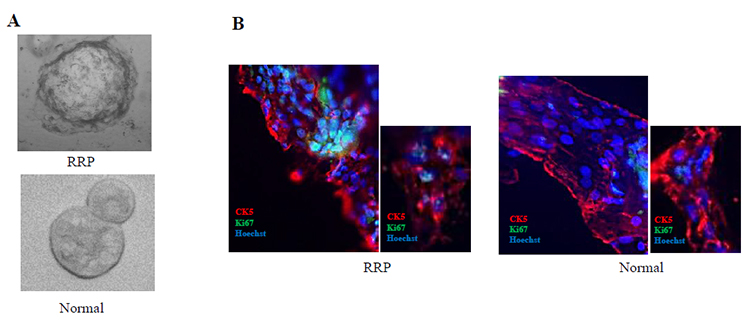
Figure 2:
Morphology and protein expression patterns of 3D culture cells derived from RRP and normal epithelium
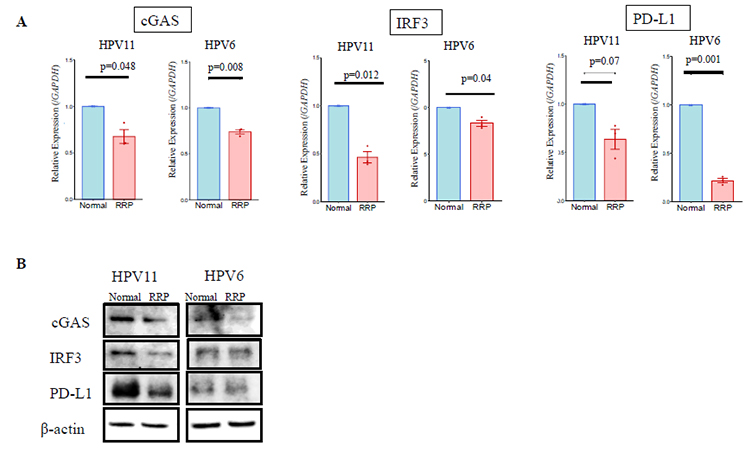
Figure 3:
Expression of innate immune factors in normal epithelium and RRP cells
Keio University Program for the Advancement of Next Generation Research Projects
The Keio University Program for the Advancement of Next Generation Research Projects subsidizes research costs with the aim of finding solutions to challenges and of promoting global academic research in order to allow Keio University faculty members to establish a presence as core researchers.