Research outcomes of the Program for the Advancement of Next Generation Research Projects
Faculty of Pharmacy
Saki Noguchi
Organic acidemia is a group of rare congenital disorders caused by the formation and accumulation of abnormal acidic metabolites due to deletions or reduced activities of metabolic enzymes for amino acids and fats. Current therapies for organic acidemia, such as low-amino acid diets and carnitine supplementation, aim to decrease the formation and enhance the elimination of organic acids. However, the efficacy of these treatments is often inadequate, highlighting the need for further research into the underlying mechanisms of the disease and disease-related symptoms.
Methylmalonic acidemia (MMA-emia) is one of the organic acidemia with the most cases of onset and is characterized by the elevated serum concentration of MMA, an abnormal metabolite of methylmalonyl-CoA. It is caused by a deficiency of methylmalonyl CoA mutase, a mitochondrial enzyme that metabolizes methylmalonyl CoA to succinyl CoA, or a defect in the synthesis of its cofactor, adenosyl-cobalamin. The symptoms of MMA-emia (e.g., vomiting, hypoglycemia, hypotonia, etc.) usually appear in early infancy, and chronic kidney disease is a characteristic long-term complication that usually appears from childhood.
MMA is mainly produced in the liver of MMA-emia patients and excreted into the urine. Renal damage in MMA-emia patients has been observed in the proximal tubules but not in the distal tubules, and its severity is correlated with serum MMA levels1-3. Furthermore, it has been reported that MMA patients who underwent liver or combined kidney and liver transplantation exhibited lower plasma MMA levels and better renal function (i.e., higher estimated glomerular filtration rate) compared to those who only underwent kidney transplantation4. Therefore, the uptake of MMA into the proximal tubular epithelia potentially causes renal toxicity in MMA-emia.
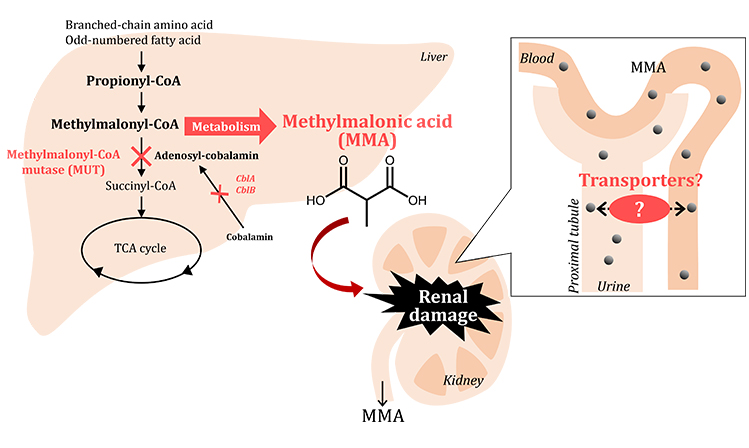
Figure 1:
The estimated mechanism of renal dysfunction in methylmalonic acidemia and the objective of the research.
©Keio University
While proximal tubule epithelial cells express many kinds of transporters, the recognition of MMA by organic anion transporters (OATs) and sodium/dicarboxylate cotransporters (NaDCs) in the kidney were expected candidates for MMA transporters. This is because MMA is estimated to mainly exist as dicarboxylate in the physiological pH. In our study we first evaluated MMA uptake by OATs and NaDCs, which are expressed in the human proximal tubules.
The uptake of [14C]MMA by OAT1-overexpressing cells was higher than that by OAT1-expression uninduced cells, indicating that MMA is an OAT1 substrate. On the other hand, cellular uptake of [14C]MMA by OAT3 was not detected. Likewise, the uptake of [14C]MMA was little affected by the overexpression of OAT4, even in the presence or the absence of extracellular Cl-. Since OAT4 uses Cl- for anion exchanges5, OAT4 is unlikely to be involved in both the uptake and efflux of MMA. With respect to NaDCs, [14C]MMA uptake activity by NaDC1-overexpressing cells was similar to that by the mock-transfected cells. Meanwhile, [14C]MMA uptake by NaDC3-overexpressing cells was higher than that by the mock cell, indicating that MMA is a NaDC3 substrate, as well.
Since OAT1 and NaDC3 are localized at the basolateral membrane of both human and rat proximal tubule epithelium, [14C]MMA uptake by rat kidney cortex slices was examined. Uptake of [14C]MMA by rat renal cortex slices was time-dependently increased, while simultaneously decreased not only by the inhibition of OAT but also by the removal of extracellular Na+. Therefore, OAT1 and NaDC3 are likely to be involved in the basolateral uptake of MMA in the proximal tubules. Furthermore, since the involvement of OAT1 was suggested in the MMA uptake in the kidney, the effect of OAT inhibitors in rats was examined. Repeatedly administering the inhibitor reduced the renal accumulation of MMA, suggesting the involvement of OAT in the renal distribution of MMA.
In conclusion, our results indicate that OAT1 and NaDC3 accept MMA as a substrate. Since these are localized at the basolateral membrane of proximal tubules, OAT1 and NaDC3-mediated uptake from the plasma may be involved in the renal accumulation and urinary secretion of MMA in MMA-emia. The results from this project open room for future research on pharmacotherapy to prevent renal failure in MMA-emia by targeting transporters.
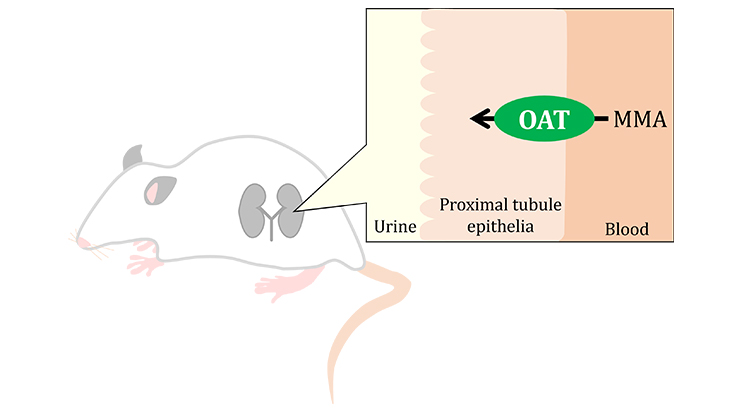
Figure 2:
The involvement of OAT was suggested in the renal distribution of methylmalonic acid in rats.
© Keio University
The Program for the Promotion of Next Generation Research Projects partially supported this work.
Reference
- Hörster F. et al., Pediatr Res. 62:225-230 (2007), 2. Zsengellér ZK. et al., Pediatr Nephrol. 29:2139-2146 (2014), 3. Kruszka PS. et al., Genet Med. 15:990-996 (2013), 4. Strologo L. et al., Mol Genet Metab. 137:265-272 (2022), 5. Noguchi S. et al., J Pharm Sci. 104:3128-3135 (2015)
Keio University Program for the Advancement of Next Generation Research Projects
The Keio University Program for the Advancement of Next Generation Research Projects subsidizes research costs with the aim of finding solutions to challenges and of promoting global academic research in order to allow Keio University faculty members to establish a presence as core researchers.