Research outcomes of the Program for the Advancement of Next Generation Research Projects
Faculty of Pharmacy
Masashi Maekawa
Skin, the outer tissue that distinguishes the inside of our body from the outside world, is the largest organ in the human body. In our daily life, the areas of the skin which are not covered by clothing are constantly exposed to a variety of stimuli, such as chemicals and ultraviolet rays, which cause DNA damage in skin tissues, especially in keratinocytes. Accumulation of DNA damage leads to age-dependent cellular senescence of keratinocytes, causing solar keratosis. Solar keratosis not only induces the development of skin cancer, but is a major cause of spots and wrinkles, which have major effects on patients' appearances, and in turn, their quality of life. The identification of the molecular mechanisms of cellular senescence of keratinocytes and its molecular signatures, especially the "lipid signature," have the potential to lead to the development of intervention methods and contribute to the development of inhibitors of skin-aging progression. Therefore, it is important to elucidate a novel molecular mechanism of cellular senescence of keratinocytes, which will be a target for the development of anti-aging agents for the prevention of skin cancer and other diseases.
Cullin-3 (CUL3) ubiquitin ligase is an E3 complex that ubiquitinates its substrate proteins via recognition receptors, BTBP. Human genome encodes 183 genes for BTBP, and since each BTBP binds to different substrate proteins, and the patterns of ubiquitin chains (the ubiquitin code) and the fates of ubiquitinated substrate proteins (degradation or addition of novel functions) are also diverse, the CUL3/BTBP axis can exert various physiological functions. SPOP (speckle-type POZ protein) is one of the BTBPs and a critical cancer-associated protein with many amino acid mutations in its substrate binding motif. The point mutations were mainly identified in prostate and endometrial cancers, and its function in tumor regulation has recently been elucidated intensively. In contrast, its function in non-cancerous cells such as keratinocytes remains largely unknown.
We have previously shown that SPOP-knockdown in human immortalized keratinocyte HaCaT cells increased p21 mRNA levels and decreased newly-DNA synthesis (Figure 1). These results suggested that SPOP-knockdown HaCaT cells exhibited G1/S arrest. Importantly, these phenotypes are quite similar to that of cellular senescence, suggesting that SPOP-depleted HaCaT cells mimic senescent keratinocytes. Additionally, cellular localization and metabolism of cholesterol were dysregulated by SPOP knockdown, suggesting that SPOP controls lipid metabolism in HaCaT cells. Thus, we hypothesized that SPOP could be a site of intervention to investigate the causal relationship between cellular senescence and changes in lipid metabolism. In the present study, we aimed to capture changes in lipid metabolism of SPOP-depleted HaCaT cells by untargeted lipidomics using LCMS-9030.
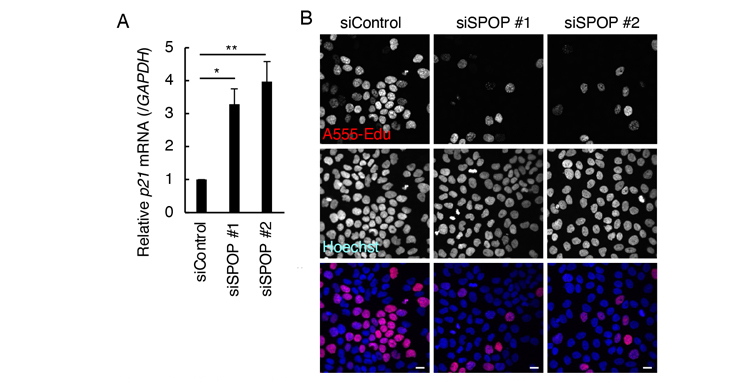
Figure 1:
SPOP knockdown upregulated mRNA level of p21 (A) and inhibited DNA biosynthesis (B) in HaCaT cells. Bars, 20μm. Figures were reproduced from Sanada et al ., BBRC. 2023
Lipid fractions extracted from SPOP-knockdown HaCaT cells, which exhibit senescence-like phenotypes, were subjected to untargeted lipidomics. We found that the amounts of major phospholipid species such as phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), and phosphatidylinositol (PI) were not affected compared to the control cells. Interestingly, the depletion of SPOP significantly decreased the amount of neutral lipids, such as triacylglycerol (TG) and diacylglycerol (DG). These results suggest that the amount of neutral lipids is decreased in cells during cellular senescence associated with G1/S arrest. Since the mRNA levels of genes related to neutral lipid biosynthesis were not affected by SPOP knockdown, the degradation of neutral lipids would be enhanced by the depletion of SPOP in HaCaT cells.
Considering our results, by inducing the degradation of neutral lipids (e.g., enforced expression of neutral lipases) without suppressing the SPOP expression and examining cellular senescence phenotypes, the causal relationships between neutral lipid metabolism and cellular senescence could be elucidated in future studies. The changes of intracellular lipid species may become novel signatures that can evaluate cell cycles and cellular senescence.
Keio University Program for the Advancement of Next Generation Research Projects
The Keio University Program for the Advancement of Next Generation Research Projects subsidizes research costs with the aim of finding solutions to challenges and of promoting global academic research in order to allow Keio University faculty members to establish a presence as core researchers.