Research outcomes of the Program for the Advancement of Next Generation Research Projects
Faculty of Pharmacy
Shunsuke Kimura
A mucous membrane lines various organs and cavities of our body that are open to the external environment, such as the ocular surface, respiratory, digestive, and reproductive tracts, and serves as a place to transmit substances and information to and from the outside. A thin epithelial layer of several tens of micrometers covers the mucosal tissue, which prevents the invasion of foreign substances by constructing a solid physical barrier, which is referred to as a "tight junction." However, this mucosal epithelial barrier does not entirely block luminal materials and information. Multiple cell lineages are specialized to either directly capture or sense luminal material as chemical and physical stimuli to appropriately transmit them to subepithelial cells, including immune cells and enteric neurons (Figure 1). For example, microfold cells (M cells) directly take up substances in the intestinal lumen and deliver them as antigens to the subepithelial immune system, activating mucosal immunity against foreign antigens. Dietary lipids and carbohydrates are not only ingested as nutrients by enterocytes but are also sensed by enteroendocrine cell, which transmits information to the enteric nervous system via hormones, and are used to regulate digestion, appetite, and metabolism.
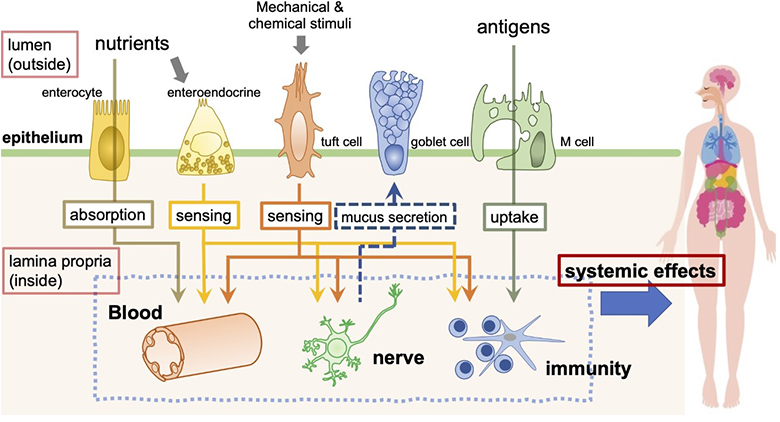
Figure 1:
The mucosal epithelium is composed of multiple specialized epithelial cells and functions as an interface for obtaining information on the mucosal surface. ©Keio University
The mucosal epithelium functions as an interface that transfers materials and information to the subepithelial immune and nervous system, and maintains the body's homeostasis. Recent studies have revealed that disruption of mucosal epithelial function leads to various systemic diseases, such as inflammation and neurological disorders. Therefore, understanding the function of the mucosal epithelium is expected to lead to the development of preventive methods for various diseases. In addition, it is thought to be helpful in controlling diseases by controlling drug delivery and information transmission into the body by bypassing physical barriers without destroying them. Therefore, in our latest research, we aimed to analyze the uptake and signal transduction mechanism in the mucosal epithelium and clarify the molecular basis of the control mechanism.
For our research, we looked at the cellular and molecular levels; specifically, we focused on M cells as a cell lineage of the mucosal epithelium and AP-1B as a molecule necessary for the function of epithelial cells.
The M cell is a specialized intestinal epithelial cell that samples luminal antigens for mucosal immune surveillance. M-cells transport macromolecules and microorganisms from the intestinal lumen into the subepithelial region via a transepithelial pathway known as antigen transcytosis (Figure 2). The transcytosed luminal antigens are subsequently phagocytosed by immature dendritic cells (DCs) residing in the subepithelial dome region, resulting in the immature DCs undergoing maturation and, in turn, activating antigen-specific naive T cells. Thus, M-cell-dependent antigen transcytosis may have an important role in the induction of the mucosal immune response to specific antigens. Taking advantage of the special properties of M cells, mucosal vaccines are being developed that target M cells and efficiently incorporate antigenic substances into the body.
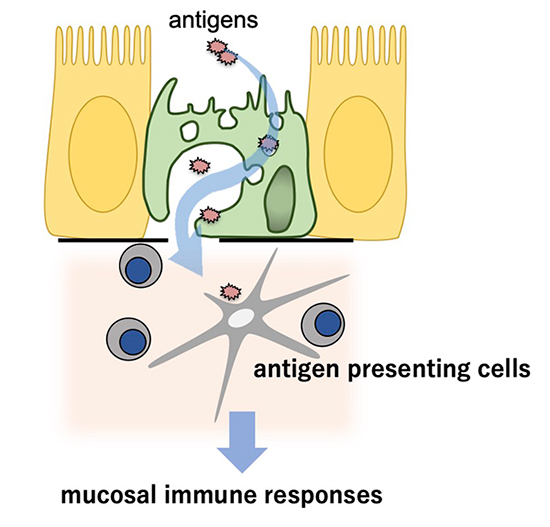
Figure 2:
M cell uptakes luminal antigens and transfers them to the antigen presenting cells underneath epithelial cells to initiate mucosal immune responses.
©Keio University
So far, research on M cells has focused on the intestinal tract, and those in other mucosal tissues has yet to progress. Therefore, using mouse models, we investigated the distribution of M cells in mucosal tissues throughout the body. As a result, we found M cells in the nasolacrimal duct, nasal cavity, and trachea (Figure 3). Interestingly, the number of tracheal M cells was significantly increased in mouse models of influenza infection, allergic disease, and chronic obstructive pulmonary disease. This result indicates that M cells may be involved in these respiratory diseases. Our study revealed that RANKL-RANKL signaling is a common signal for M-cell differentiation in systemic mucosal tissues. We found that M-cell progenitor and inducer differed depending on the mucosal tissue. Using genetic engineering, creating mice lacking M cells in specific mucosal tissues will be possible. This could be useful in clarifying the function of M cells in mucosal tissue.
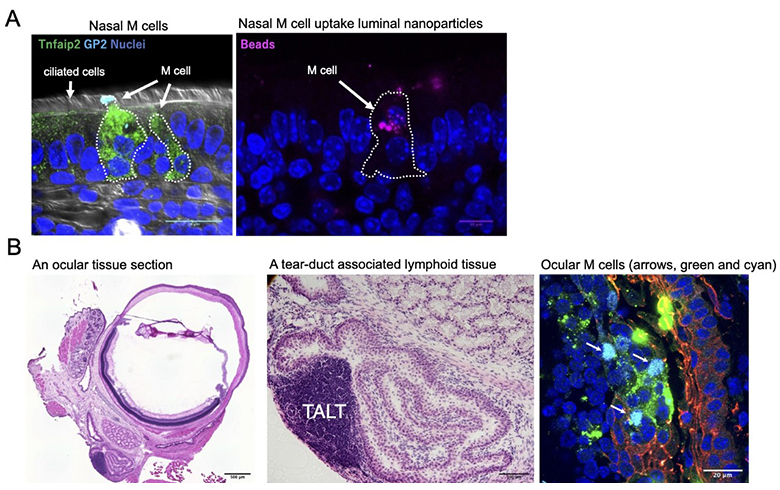
Figure 3:
(A) Nasal M cells marked with Tnfaip2 (green) and GP2 (cyan) can uptake luminal nanoparticles (right panel, magenta). (B) A murine ocular tissue section. A teat-duct associated lymphoid tissue (TALT) is present in the nasolacrimal duct. Ocular M cells (arrows in the right panel) are found on the follicle associated epithelium covering the lymphoid follicles of TALT.
©Keio University
The intestinal epithelium is polarized and divided into the apical and basolateral basal surfaces, separated by tight junctions. Intestinal epithelial cells are in contact with interepithelial lymphocytes (IELs) on the basolateral surface to protect against foreign pathogens and maintain epithelial barrier functions. AP-1B is a clathrin adaptor protein (AP) complex specifically expressed in polarized epithelial cells and required for efficiently sorting several membrane proteins to the basolateral membrane. This proper sorting is thought necessary for the functions of epithelial cells; however, experimental evidence still needs to be provided. We found that AP-1B is required for the interaction between the intestinal epithelium and interepithelial lymphocytes and further identified a new molecule involved in the interaction between epithelium and IEL. This discovery of a new function of AP-1B is expected to lead to the discovery of a new regulatory mechanism in the host defense of the intestinal epithelium.
Our research provides new insights into the interaction between the intestinal epithelium and the immune system. With further progress in this research, we aim to redefine life phenomena from the new perspective of regulation of homeostasis by the mucosal epithelial interface and bring about innovations in pharmaceutical science and medicine.
Keio University Program for the Advancement of Next Generation Research Projects
The Keio University Program for the Advancement of Next Generation Research Projects subsidizes research costs with the aim of finding solutions to challenges and of promoting global academic research in order to allow Keio University faculty members to establish a presence as core researchers.