Research outcomes of the Program for the Promotion of Next Generation Research Projects
School of Medicine
Jin Nakahara
The 21st century is said to be the age of the brain, and both fundamental neuroscience and clinical therapeutics have made remarkable progress. Despite this progress, brain science is still in its developmental stage. For instance, innovative advances in diagnostic imaging techniques and endovascular therapy have greatly improved treatment outcomes for ischemic stroke patients. Still, it is not easy to clinically apply the results of basic research, such as stem cell therapy, to improve the functional prognosis of stroke patients. The main reason is that the functions of each cell, tissue construction through connections, and functional interaction of various tissues of the brain have not been fully elucidated.
The brain is composed of approximately 14 billion neurons and ten times as many glial cells. The conceptual framework of the smallest functional unit consisting of neurons, cerebral blood vessels, and glial cells is called a Neurovascular Unit (NVU). Elucidating the function of the NVU and its involvement in pathological conditions is expected to open new doors in brain science.
In this program, we conducted fundamental research focused on the function of each cell composing the NVU using experimental animals. The program sought to elucidate the functions of cerebral cortical microstructures by combining three principal techniques: the creation of a chronic cranial window that allows repeated observation of the mouse brain in vivo (Figure 1), fluorescent labeling of specific cells comprising the NVU (neurons, cerebral vascular endothelial cells, and glial cells), and long-term repeated in-vivo observation of the cortex through the cranial window using two-photon microscopy.
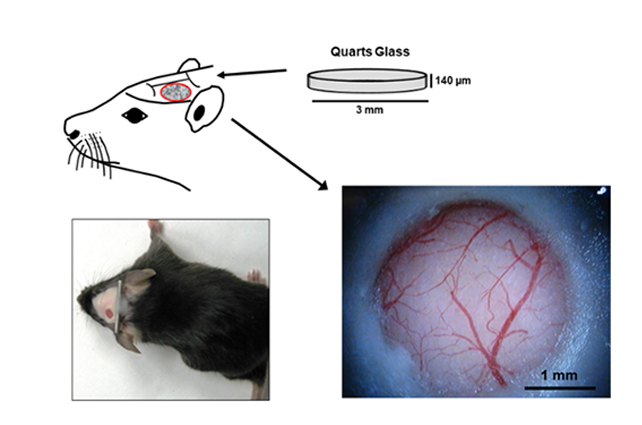
Figure 1.
Chronic cranial window using the Tomita-Seylaz technique. A portion of the temporal parietal bone was excised and a quartz glass was implanted to allow long-term repeated observation of the cerebral cortex in-vivo (Tomita Y. et al, J Cereb Blood Flow Metab 25:858-67, 2005).
Project 1: Establishment of a Model for Analysis of Increased Vascular Permeability and Analysis
An increase in cerebral vascular permeability is implicated in the onset and exacerbation of many neurological diseases, such as cerebral infarction, vascular dementia, and brain metastasis of cancer cells. However, the mechanism by which cerebral vascular permeability is altered has not yet been fully elucidated. This program has established a method to continuously track fluorescent dextran, which does not leak across the blood-brain barrier in the normal brain, outside of blood vessels under certain conditions using two-photon microscopy. With this model, we have succeeded in continuously capturing changes in the permeability of cerebral cortical vessels due to exposure to vasoactive substances or artificial cerebral infarction caused by middle cerebral artery occlusion.
Project 2: Manipulation of NVU Constitutive Cells and its Effects on Function
We used transgenic mice expressing the light-sensitive protein channelrhodopsin 2 (ChR2) in neurons, glial cells, pericytes, or vascular smooth muscle, which are the component cells of NVU (Figure 2). By irradiating these mice with blue light to specifically activate each cell, we found that the activation of astrocytes or neurons increased blood flow through different mechanisms.
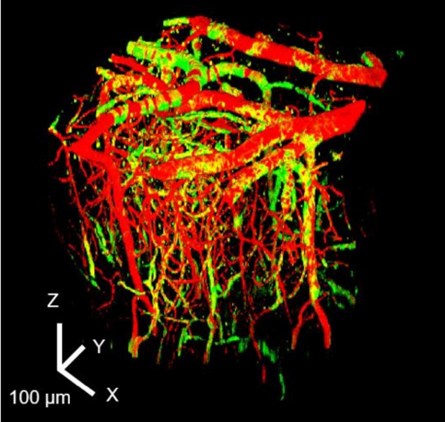
Figure 2.
Three-dimensional structural images representing blood vessels (sulforodamine 101; red) and ChR2 (co-expression with YFP; green) expressed in vascular smooth muscle cells and vascular pericytes in the cerebral cortex (0-400 μm).
Project 3: Elucidation of the Effects of Brain Edema on NVUs
The water channel aquaporin 4 (AQP4) is expressed in astrocytes that construct NVU. AQP4 is involved in water movement in brain tissue, including brain edema formation. In this study, we evaluated the involvement of AQP4 in edema formation in a water intoxication model. AQP4 deficient mice showed reduced brain edema, reduced astrocyte swelling, and perivascular space reduction.
Project 4: Elucidation of the Involvement of Cortical Spreading Depression (CSD) in Neurological Disorders
CSD is a phenomenon in which mass depolarization of neurons and glia in the cerebral cortex is propagated to the surrounding area and sustainably suppresses the electrical activity of neurons. CSD has been suggested to play a role in some neurological disorders, such as migraines and subarachnoid hemorrhage. Here, the effect of CSD on capillary hemodynamics was investigated: analysis of red blood cell velocity through capillaries after CSD induction revealed an increase in the velocity during neuronal activation. Furthermore, focusing on the possible association between CSD and migraine aura, the effects of CSD were examined in a transgenic mouse model of familial hemiplegic migraine. The results revealed that migraine model mice, compared to normal mice, are more sensitive to CSD inducible stimulation and that CSD affects a broader brain area.
In addition, to strengthen domestic and international collaboration in NVU research, we hold the NVU Workshop once a year, inviting leading experts in brain research from Japan and abroad to discuss the latest topics in NVU research. This workshop is supported by the Next Generation Research Project Promotion Program. Furthermore, as an extension of this workshop, the international U.S.-Japan Joint Workshop on Neurovascular Units was selected as a grant program of the U.S.-Japan Science and Technology Cooperation Program jointly promoted by the National Institutes of Natural Sciences, Japan, and the National Institutes of Health, USA. This workshop will next be held on January 6-9, 2023, at Keio University's Mita Campus.
This project helps elucidate cell-tissue-organ relationships and the pathogenesis of neurological diseases from the perspective of NVU. It has also created an environment where basic and clinical research can be conducted with an integrated approach. We will continue to develop this program so that the NVU Workshop will become a core research hub for medicine-engineering, industry-academia, and international cooperation.
Reference
- Hatakeyama N, Unekawa M, Murata J, et al., Differential pial and penetrating arterial responses examined by optogenetic activation of astrocytes and neurons. J Cereb Blood Flow Metab. 2021:41:2676-2689.
- Unekawa M, Tomita Y, Toriumi H, et al., Spatiotemporal dynamics of red blood cells in capillaries in layer I of the cerebral cortex and changes in arterial diameter during cortical spreading depression and response to hypercapnia in anesthetized mice. Microcirculation. 2019:26:e12552.
- Tang C, Unekawa M, Shibata M, et al., Characteristics of cortical spreading depression and c-Fos expression in transgenic mice having a mutation associated with familial hemiplegic migraine 2. Cephalalgia. 2020:40:1177-1190.
Keio University Program for the Promotion of Next Generation Research Projects
The Keio University Program for the Promotion of Next Generation Research Projects subsidizes research costs with the aim of finding solutions to challenges and of promoting global academic research in order to allow Keio University faculty members to establish a presence as core researchers.