Research outcomes of the Program for the Promotion of Next Generation Research Projects
Koji Hase
Faculty of Pharmacy and Graduate School of Pharmaceutical Science
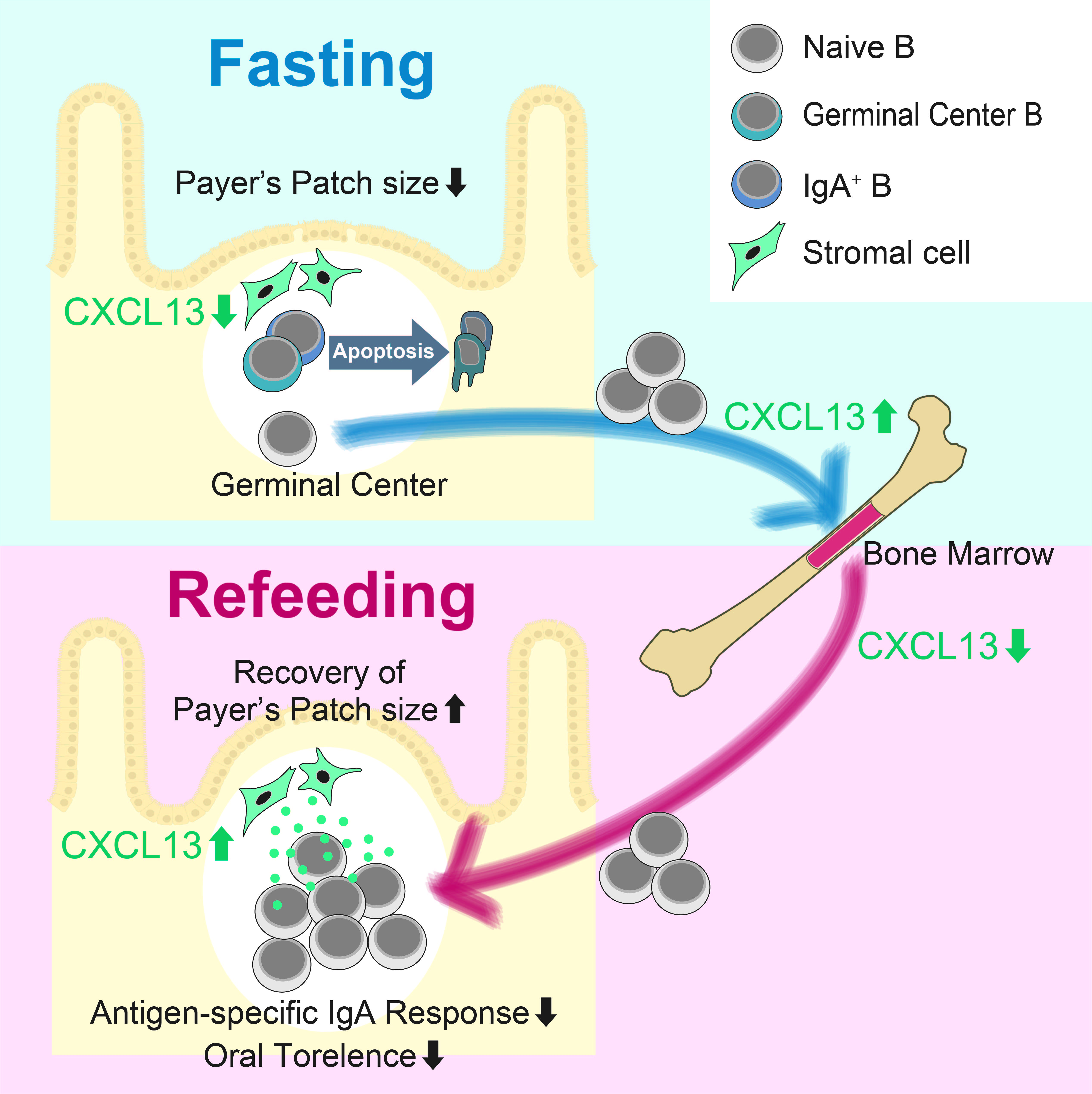
Figure A. B cell dynamics during fasting and refeeding. During fasting, naïve B cells egress Peyer's patch to migrate to the bone marrow. Meanwhile, GC B cells undergo apoptosis. Refeeding induces homing of naïve B cells to Peyer's patch. ©2019 Keio University
Inappropriate food intake is emerging as a global health concern. In developing countries, malnutrition often compromises immune responses, leading to the reduced efficacy of vaccination and the increased risk of infectious diseases. Meanwhile, in advanced countries, excess food intake with less exercise has been implicated in the low-grade inflammation leading to metabolic syndrome and cancer. These observations indicate that nutritional status has a significant impact on the immune system. However, it remains obscure how the nutrient signals regulate the immune cell dynamics and functionality.
We previously demonstrated that intestinal tissue is highly susceptible to deprivation of luminal nutrients; fasting caused cell arrest and refeeding induced hyperproliferation in the intestinal epithelium (1). We here analyzed the impact of fasting-refeeding on cellular dynamics and functions of the gut immune system. We observed that 36 h of fasting drastically decreased the number of lymphocytes in Peyer's patch, and subsequent 72 h of refeeding per se restored the immune cells (2). However, the cellular composition was conspicuously altered by fasting-refeeding. Naïve B cells migrated to the bone marrow at the fasting period, and back to Peyer's patch at the refeeding stage (Figure A). Similar oscillation was also evident to a lesser extent even in ad libitum fed mice with naïve B cells shuttling Peyer's patch and the bone marrow during nocturnal (active eating) and daily (resting) time, respectively.
In sharp contrast, germinal center B and IgA+ B cells were not recovered due to massive apoptosis during the fasting. Consequently, the lymphoid follicles were replenished by naïve B cells and the frequency of the memory-like B cell subsets prominently decreased after the fasting-refeeding treatment. Eventually, fasting-refeeding treatment attenuated antigen-specific IgA response to oral immunization with ovalubmin (OVA) (Figure B). Notably, the fasted group are highly susceptible to OVA-induced food allergy, due to reduced production of OVA-specific IgA that protects infiltration of the allergen into the body as well as the abrogation of oral tolerance to OVA.
We also investigated the molecular basis of naïve B cell dynamics in response to nutrient-sufficient and deficient conditions. We found that expression of CXCL13 essential for B cell migration is significantly downregulated in Peyer's patch of fasted mice, whereas it was upregulated in the bone marrow (Figure A). In peripheral lymph node-derived stromal (BLS12) cells, CXCL13 is induced by stimulation with TNF-α and anti-LTβR agonistic antibody. Interestingly, these cytokine signalings skew the metabolic status of BLS12 cells toward glycolysis-dominant energy generation, the reminiscent of Warburg effect. In agreement with this observation, inhibition of glycolysis by 2-deoxy-D-glucose markedly downregulated the gene expression of Cxcl13, without affecting Cxcl12.
In conclusion, we found that food intake secures the integrity and functions of the gut mucosal immune system through nutritional signaling (2). The deprivation of nutrition impairs mucosal immunity, leading to immune barrier dysfunction, and excessive allergic response. Our study uncovers the novel link between the nutritional signals and the immune cell dynamics and functionality. Furthermore, these finding may open up a new research direction to enhance vaccine efficacy via dietary intervention.
Figure B. OVA-specific lgA titers in feces from mice under ad libitum- fed(black dots) or fasting-refed conditions. Mice were orally immunized with ovalbumin(OVA) and cholera toxin(CT) once a week for 4 weeks. ©2019 Keio University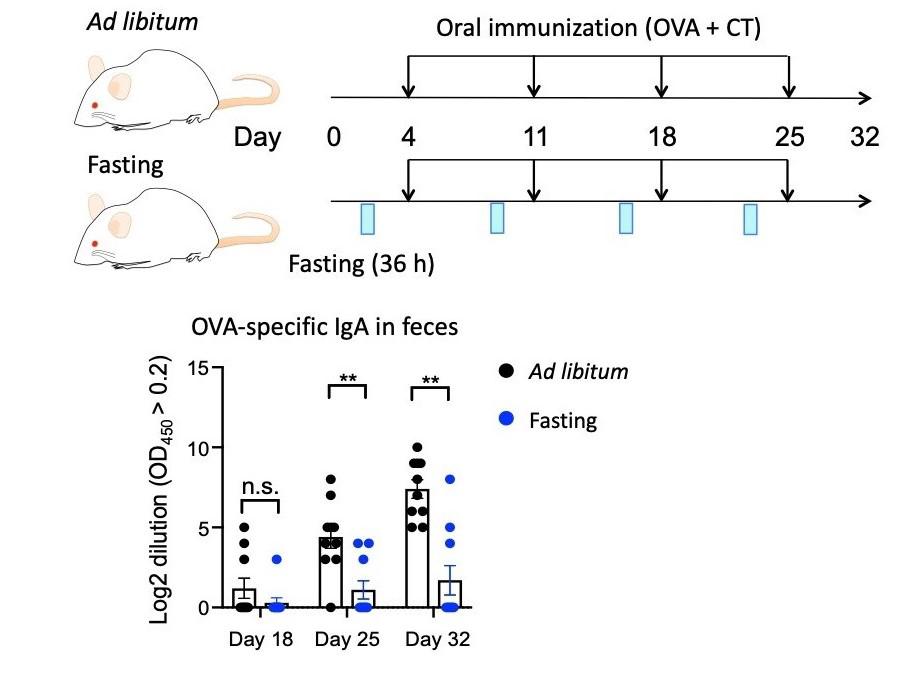
1. T. Okada et al., Microbiota-derived lactate accelerates colon epithelial cell turnover in starvation-refed mice. Nat. Commun. 4, 1654 (2013).
2. M. Nagai et al., Fasting-Refeeding Impacts Immune Cell Dynamics and Mucosal Immune Responses. Cell. 178, 1072-1087 (2019).
Keio University Program for the Promotion of Next Generation Research Projects
The Keio University Program for the Promotion of Next Generation Research Projects subsidizes research costs with the aim of finding solutions to challenges and of promoting global academic research in order to allow Keio University faculty members to establish a presence as core researchers.