Research outcomes of the Program for the Promotion of Next Generation Research Projects
Kosuke Tsukada
Faculty of Science and Technology
Prophylactic vaccines used for mass vaccination programs rely on purified or recombinant antigens from pathogens as immunogens. However, these immunogens do not effectively induce immune responses on their own and thus cannot sufficiently protect against infection. Owing to this, most of the current vaccines are supplemented with chemical or biological immunostimulants, known as vaccine adjuvants, to enhance immune responses. Although vaccine adjuvants have strong effects on innate immunity activation, there is a significant concern regarding adverse reactions because of their reactogenicity and long-term retention in the biological system. Some studies have suggested that conventional adjuvants could be linked to serious adverse reactions such as injection site pain and inflammation, central nervous system damage, fatigue, and myalgia, all of which can lead to anxiety and a lack of public trust in vaccine safety. Hence, social demands have risen for the development of safe and effective vaccine adjuvants.
Our research team has proposed a laser adjuvant technology that involves short-term and low-dose irradiation of the vaccination site with near-infrared lasers and demonstrated that such a laser adjuvant augmented the influenza vaccine effects without causing apparent adverse reactions as compared with that of a conventional chemical vaccine adjuvant. Unlike chemical adjuvants, laser light does not remain in the body; therefore, laser adjuvant is expected to be associated with less adverse reactions. Because laser adjuvant technology can be combined with vaccines without creating an irreversible mixture, it could be applied to any current or candidate vaccine. Moreover, a single piece of laser equipment can be repeatedly used to treat numerous individuals undergoing vaccination, making laser adjuvant technology cost-efficient and likely to reduce the unit cost of vaccination. This project's specific achievements are summarized below.
Mechanisms of immune activation using laser adjuvant
Because mast cells and dendritic cells play important immune response roles, we hypothesized that these cells were involved in the adjuvant effect of near-infrared laser irradiation. We used a mouse model of influenza vaccination to investigate laser adjuvant mechanisms. The mouse skin was treated with a continuous wave near-infrared laser and intradermally injected with a model influenza vaccine. The skin-resident migrating dendritic cell population, particularly the Lang+CD103+ and CD11b−Lang− subsets, and CCR2+ inflammatory dendritic cells were specifically activated by laser irradiation. We further demonstrated that the laser treatment augmented dendritic cell migration into the regional lymph nodes, thereby amplifying antibody production against the vaccine. Additionally, we revealed that reactive oxygen species transiently produced at the irradiated site stimulated mast cells and induced expression of a limited set of cytokines without causing overt inflammation, selectively activating the skin-resident dendritic cells. These insights into immune activation mechanisms indicated that near-infrared laser irradiation was a safe and effective method for enhancing the immune response in the skin.
Development of laser adjuvanting devices
For clinical use, we built inexpensive, handheld laser equipment comprising a semiconductor diode that emitted near-infrared laser light with wavelengths of 1061, 1258, and 1301 nm. Using this equipment in a mouse model of influenza vaccination, we demonstrated that our equipment could augment the immune response to the vaccine as effectively as an expensive, conventional diode-pumped solid-state laser device. Developing an economical small laser device is an important step toward clinical application of laser adjuvant technology for mass vaccination programs and various medical procedures.
Visualization of vaccine dynamics
To understand the laser adjuvant's mode of action, technology to analyze biodistribution of vaccine antigen in vivo in real time is desirable. However, there is a paucity of high-resolution imaging methodology in this area. To respond to this unmet need, we have developed a vaccine tracking system that allows longitudinal biodistribution monitoring of administered vaccine antigens. We have created model vaccine antigens labeled with a zwitterionic near-infrared heptamethine fluorophore ZW800-1C, which has minimal interaction with biological tissues. Mice were imaged under multispectral near-infrared fluorescent imaging after undergoing intradermal vaccination with the model influenza vaccine. This system visualized vaccine translocation through the lymphatic system into the draining lymph nodes in real time with high signal-to-background ratio. These results demonstrated that our zwitterionic fluorophore-based near-infrared imaging system was a powerful tool to describe vaccine antigen dynamics in vivo. This method is expected to be broadly used by researchers in this field, and its detailed protocol has been published by our group.
These findings suggest that the laser adjuvant technology is a simple, effective, and safe alternative to chemical and biological adjuvants, which have been implied to have adverse reactions to vaccines. Additionally, unlike visible light, near-infrared light is not affected by skin color, making it applicable to patients irrespective of their race. The handheld laser device can also be economically produced and repeatedly used in mass vaccination upon recharging and thus is expected to reduce the unit cost of vaccination. Such a technology would likely increase access to vaccination programs in developing countries.
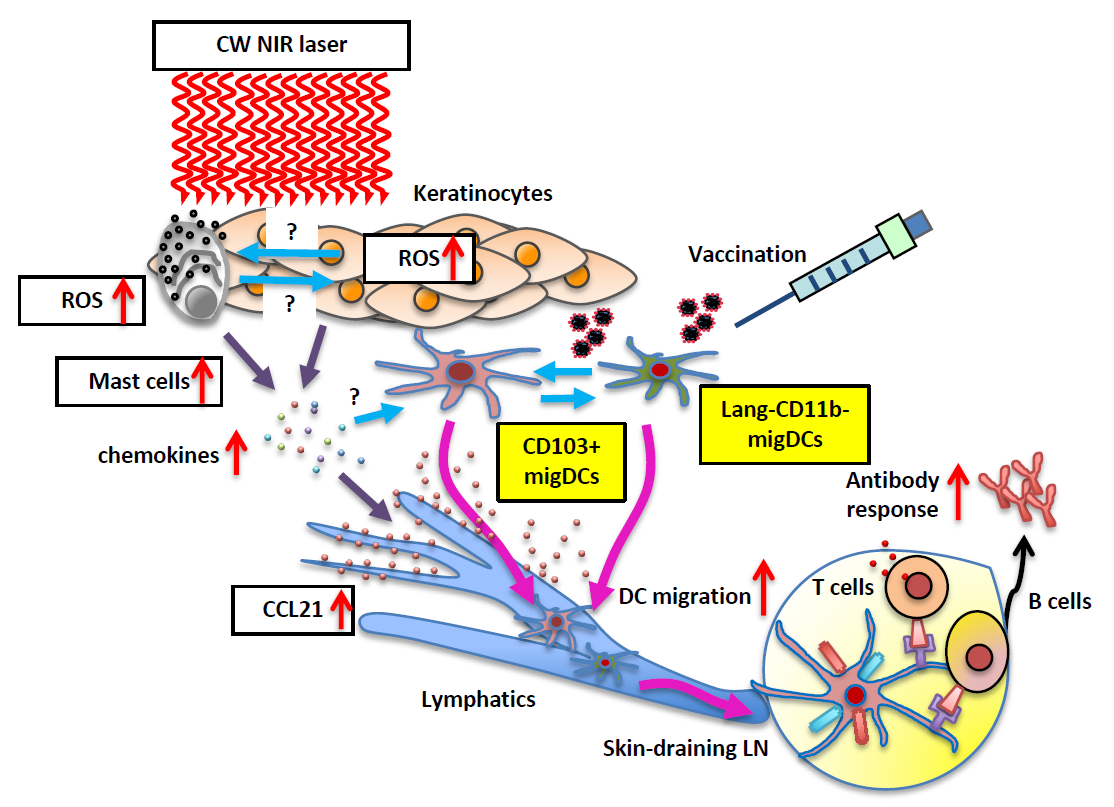
Schematic diagram of the mechanism of action of the CW NIR laser adjuvant. ©2019 Keio University
Keio University Program for the Promotion of Next Generation Research Projects
The Keio University Program for the Promotion of Next Generation Research Projects subsidizes research costs with the aim of finding solutions to challenges and of promoting global academic research in order to allow Keio University faculty members to establish a presence as core researchers.