Researchers develop a method for simultaneously mapping oxygen level and spatially uneven vessel permeability in tumor tissue
Published online 30 October 2018
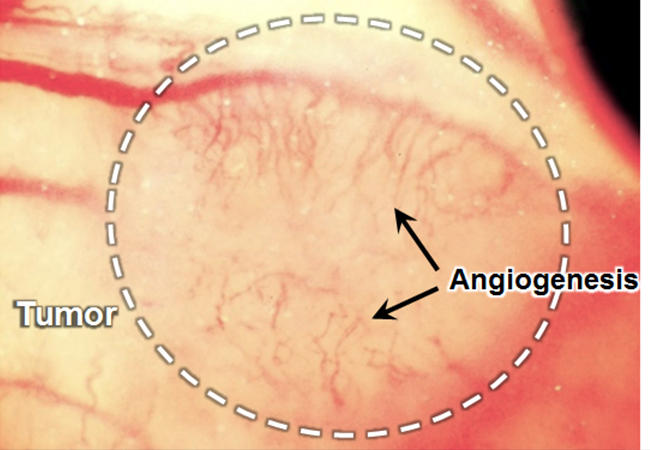
Tumor angiogenesis (black arrows) in murine breast cancer (white circle) grown on a mouse back skin © Kosuke Tsukada, Keio University
In tumors, newly formed blood vessels tend to have an abnormal tissue distribution -- vessel permeability and oxygen delivery are spatially more uneven than normal. To better understand tumor mechanisms and to optimize anticancer drug delivery strategies, it is important to be able to quantify both blood vessel permeability and local hypoxia (oxygen deficiency). Kosuke Tsukada and colleagues at Keio University have now developed a method enabling the simultaneous measurement of both permeability and hypoxia in tumor environments.
The researchers imaged the flow of blood, made fluorescent, in tumors implanted in mice. The mice were put on the stage of a confocal laser scanning microscope (a type of microscope used for reconstructing 3D structures) and after injecting a fluorescent dye, fluorescence images were recorded every minute. Image processing techniques were then applied to quantify vessel permeability and how it changes over time. (Fluorescence was excited by irradiation with a continuous-wave laser.)
During the permeability measurement process, for the same field of view of the microscope, the tissue oxygen partial pressure (a measure for the presence of oxygen) was quantified through phosphorence. A phosphorent substance was injected in the mice, after which the phosphorence lifetime was measured; the latter can be related to oxygen concentration. (In contrast to fluorescence, phosphorence was triggered by a pulsed laser.)
The oxygen partial pressure was found to be higher in normal tissue than in tumor tissue; in the latter, vessel networks were generally more complex. Regarding vessel permeability, the scientists found that for normal tissue it was generally evenly distributed, whereas for tumor tissue there was greater heterogeneity.
Previous methods could only achieve mean results for tissue regions or for the entire tissue, and combining oxygen partial pressure mapping with permeability measurements was not possible at all. This demonstration by Tsukada and colleagues shows that it is feasible to simultaneously measure tumor vessel permeability and oxygen partial pressure in vivo. The scientists believe that "[their] findings could be applied to improve anticancer drug delivery and radiotherapy by identifying the dependence of local tissue oxygenation on the vessel structure and hemodynamics," and anticipate that "this method can be applied to investigate other issues, such as visualization of the relationship between parietal cell dynamics [(processes taking place in the stomach)] and vessel permeability caused by hypoxia."
About the researcher

Kosuke Tsukada― Associate Professor
Department of Applied Physics and Physico-Informatics, Faculty of Science and Technology
Kosuke Tsukada is an associate professor at Keio University. He received his Ph.D. from Keio University in 2002. He joined the Edwin L. Steele Laboratory for Tumor Biology (Prof. Rakesh K. Jain) in Massachusetts General Hospital and Harvard Medical School from 2007 to 2009. His laboratory studies optical and imaging techniques for tumor microcirculation.
Links
Reference
- Oda, K., Iwamoto, Y. & Tsukada, K. Simultaneous mapping of unevenly distributed tissue hypoxia and vessel permeability in tumor microenvironment. Biomed. Phys. Eng. Express 2, 065017 (2016). | article