From sensitive sensors to carbon dioxide recycling, diamond with a smattering of boron atoms has a diverse range of applications
Published online 24 April 2018
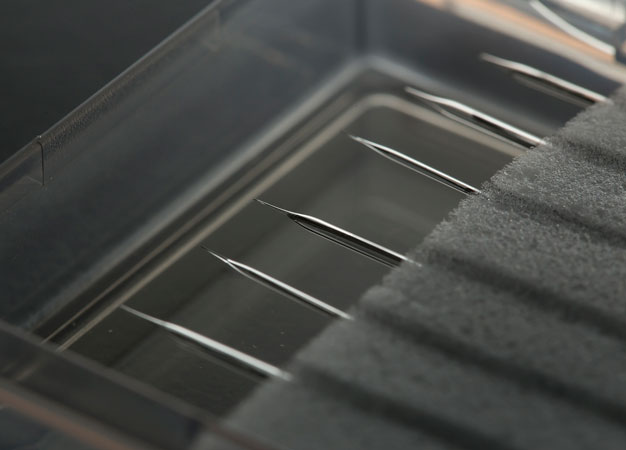
Conductive diamond in the form of needle-like microelectrodes makes excellent biocompatible sensors.
© 2018 Yasuaki Einaga
There is more to diamond than its sparkle. The material's exceptional hardness and thermal conductivity have seen it be used extensively in industry. And modifying diamond's structure can make it even more useful. For example, adding boron atoms turns diamond into a good conductor of electricity. Such boron-doped diamond (BDD) is finding uses as wide ranging as wastewater treatment, biomedical sensing, and conversion of carbon dioxide emissions into useful chemicals.
For the past 17 years, Yasuaki Einaga from Keio University's Department of Chemistry has been exploring BDD's properties and pioneering new potential uses. Einaga and his team have shown that BDD is ideal for making electrodes. As well as combining high electrical conductivity with exceptional hardness and stability, "diamond electrodes show highly interesting and superior electrochemical properties compared to conventional electrodes," Einaga explains. "They have great potential as next-generation functional materials."
Strength and sensitivity
Einaga initially focused on developing BDD electrodes for electrochemical sensors. These sensors exploit the fact that many substances undergo electrochemical oxidation at a characteristic voltage. When the analyte, such as a heavy-metal ion in a water sample, is present, an easily detectable spike in current occurs at that voltage. "The sensitivity is good because of the surface inertness of diamond," says Einaga. "Because the background current is very small, the ratio of the signal to the background is high." The team is currently collaborating with the private sector to develop various commercial sensors, including heavy-metal ones.
Being made of carbon, diamond electrodes are highly biocompatible and have many potential biomedical applications. In a recent animal study, Einaga co-led a team that used a BDD microelectrode sensor to monitor the concentration of a drug in the body in real time following intravenous injection1. The system provided time-resolved, tissue-specific data that reveals the drug's effects with unprecedented detail.
Recycling carbon dioxide
The array of potential uses for BDD is so broad that Einaga's research has diversified far beyond sensors. One area is to use the BDD electrode as a stable, highly efficient electrochemical catalyst for organic synthesis. In particular, Einaga is focusing on BDD's ability to electrochemically reduce carbon dioxide ― the biggest contributor to global warming ― to convert the waste gas into useful products2. "Forming useful compounds such as alcohols, aldehydes or formic acid by electrochemically reducing carbon dioxide is a very important and urgent topic," Einaga says. Electrochemical reduction has the potential to become a large-scale method for recycling carbon dioxide emissions.
The team is also developing BDD electrodes for generating ozone ― an environmentally friendly disinfectant used in hospitals, for example ― as a way to break down harmful compounds in wastewater3. "I can no longer say what is the main application we study in our lab, because we are studying all these fields in parallel," Einaga notes.
After 17 years, diamond research continues to hold great appeal for Einaga. "I am still fascinated by this interesting material, which has many faces ― jewelry, semiconductors, superior electrodes ― in spite of its simple structure."
About the researcher

Yasuaki Einaga― Professor
Department of Chemistry, Faculty of Science and Technology
Yasuaki Einaga received his BS (1994), MS (1996), and PhD (1999) from the University of Tokyo. After 2 years as a research associate at the University of Tokyo, he started a faculty career as an assistant professor in Keio University in 2001, where he was promoted to professor in 2011. He has been also a research director of JST-CREST (2011-2014), and JST-ACCEL (2014-present). He was awarded "the Chemical Society of Japan Award for Creative Work" in 2016 for his pioneering work in diamond electrodes. His research interests include functional materials science, photochemistry, and electrochemistry.
Links
References
- Ogata, G. et al. A microsensing system for the in vivo real-time detection of local drug kinetics. Nature Biomedical Engineering 1, 654-666 (2017). | article
- Natsui, K., Iwakawa, H., Ikemiya, N., Nakata, K. & Einaga, Y. Stable and highly efficient electrochemical production of formic acid from carbon dioxide using diamond electrodes. Angewandte Chemie International Edition 57, 2639-2643 (2018). | article
- Einaga, Y. Electrochemical Application of Diamond Electrodes. In V. K. Sarin (Editor-in-Chief) & C. E. Nebel, Comprehensive Hard Materials 3, 493-512. Elsevier (2014). | article