Revelation of how a spider toxin binds to a membrane protein could lead to new drug therapies
Published online 29 September 2017
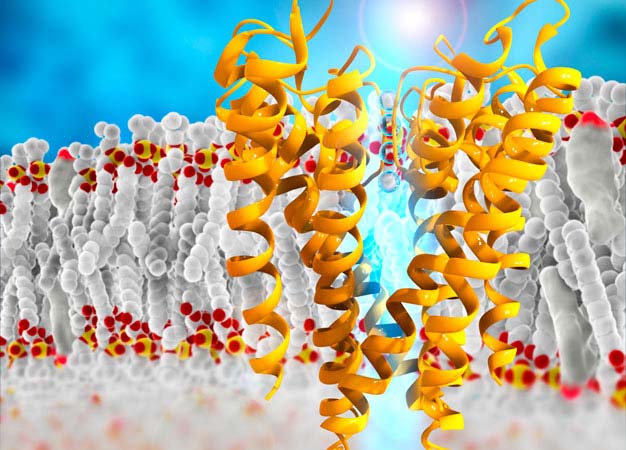
Schematic image showing the molecular structure of a potassium channel in the cell membrane.
© RAMON ANDRADE 3DCIENCIA/SCIENCE PHOTO LIBRARY
A structural analysis of how a toxin from tarantula venom disrupts electrical signaling proteins could help pave the way for new drugs to treat cancer, heart disease, neurological disorders, and other conditions.
The human genome encodes 40 different voltage-gated potassium ion channels (Kv), each of which allows potassium ions to flow across the cell membrane (see image). This generates electrical signals in cells that are involved in everything from neurotransmission to muscle contraction.
The Kv proteins consist of two domains that differ in function and structure: a pore that conducts ions and a sensor that reads the electrical gradient across the membrane. Toxins used by poisonous animals act by either blocking the pore or modifying the sensor. These two mechanisms are also promising for developing new drugs.
In theory, both domains offer potential drug targets, but completely shutting off the channels causes major problems such as heart arrhythmias. Consequently, most researchers ― including Keio University's Masanori Osawa ― are trying to find ways to fine tune the activity of the voltage-sensing domain (VSD), which acts as a gatekeeper for the ion pore.
"The VSD is a fascinating drug target portion in Kv channels," says Osawa, a structural biologist at the Keio University Faculty of Pharmacy, "but no structural information had been obtained on the interaction between the VSD and a gating modifier toxin."
To rectify this, Osawa teamed up with scientists at the University of Tokyo to look at how a toxin found in the venom of the Chilean Rose tarantula inhibits Kv channels by binding to the VSD1. Using a nuclear magnetic resonance method that the group had previously developed2, the researchers identified the precise binding sites. They then used this information to build a structural model of how the toxin docks onto the domain and blocks the protein's function.
Their model indicates that the toxin stabilizes the VSD in a certain conformation, locking it in that position. "This stabilization provides a possible mechanism for a new drug targeting the VSD," says Osawa.
Other toxins known to bind the domain ― such as those found in the venoms of scorpions, snakes, and sea anemones ― probably connect at different sites to induce other conformations, Osawa notes. These too could provide a treasure trove of therapeutic drug leads and warrant study in greater structural detail, he says.
"We are only just beginning to understand the structural basis of how gating modifier toxins bind the VSD," Osawa says.
About the researcher

Masanori Osawa ― Professor
Faculty of Pharmacy/Department of Pharmaceutical Sciences
Masanori Osawa has been researching the structural mechanisms of protein functions at the University of Maryland (1999-2003), the University of Tokyo (2003-2015) and the Faculty of Pharmacy at Keio University (2015-present).
Links
References
- Ozawa, S., Kimura, T., Nozaki, T., Harada, H., Shimada, I. & Osawa, M. Structural basis for the inhibition of voltage-dependent K+ channel by gating modifier toxin. Scientific Reports 5, 14226 (2015). | article
- Igarashi, S., Osawa, M., Takeuchi, K., Ozawa, S. & Shimada, I. Amino acid selective cross-saturation method for identification of proximal residue pairs in a protein−protein complex. Journal of the American Chemical Society 130, 12168-12176 (2008). | article