Cancer cells adapt to genomic abnormalities by producing a protein that confers drug resistance
Published online 31 August 2017
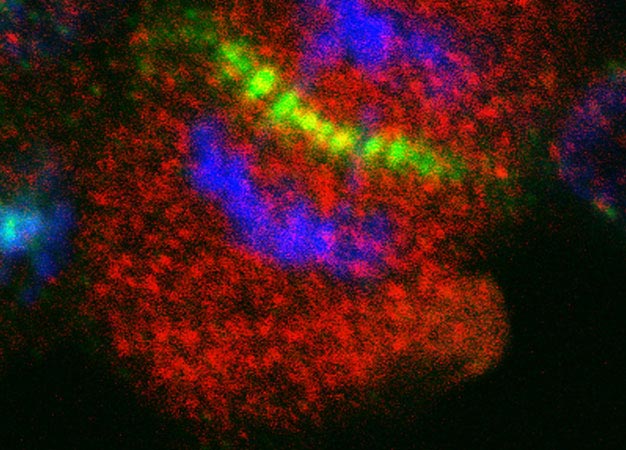
Fluorescence imaging of aurora kinase inhibitor-resistant cells reveals that aurora kinase B (green) and AKT3 (red) overlap at the center of the cell during cell division. The blue label indicates chromosomes.
© 2017 The American Society for Biochemistry and Molecular Biology
Resistance is a vexing problem in the world of cancer therapy, with many patients either failing to respond or benefiting only temporarily from a given drug. A recent study by Kohji Noguchi and colleagues at Keio University has uncovered a survival mechanism that tumor cells use to elude destruction, and a potential avenue for designing more effective cancer treatments1.
Drugs that sabotage cell division, or mitosis, can help to slow down and stop tumor growth. "Classical anticancer drugs, such as vinca alkaloids and taxanes, target mitosis," explains Noguchi. "And mitotic aurora kinases have also been considered as a novel pharmacological target." These enzymes help manage the appropriate redistribution of genetic material during cell division, and several aurora kinase inhibitors are showing promise in cancer clinical trials. "However, we lack predictable biomarkers for these inhibitors to understand their effectiveness," says Noguchi.
Noguchi and his colleagues therefore exposed colorectal cancer cells to increasing doses of an aurora kinase inhibitor, VX-680, in order to isolate a variety of drug-resistant clones for closer study. One effect of aurora kinase inhibitors is the accumulation of abnormal numbers of chromosomes, a state known as aneuploidy. Aneuploidy is often observed in cancer cells, but this condition typically triggers a self-destruct mechanism in healthy cells. The researchers observed that their VX-680-resistant cells had become highly aneuploid, containing 70-80 chromosomes rather the normal complement of 46, but they had clearly acquired a mechanism that allowed them to survive despite their condition.
A closer examination revealed that four of these five drug-resistant cell lines were producing excessive levels of a signaling protein called AKT3. A series of experiments in other cell lines subsequently provided compelling evidence that AKT3 prevents both the cell death and chromosomal abnormalities caused by aurora kinase inhibition. According to Noguchi, this was unexpected. "The AKT family has multiple oncogenic functions and is usually considered to work in cell survival mechanisms, which would provide resistance to cytotoxic drugs," he says. "However, there were no reports connecting AKT with aneuploidy."
Aneuploidy is not uncommon in malignant cells, and Noguchi believes that these results reveal a connection between this characteristic and the emergence of drug resistance via AKT3. This highlights an important potential escape mechanism for tumors being treated with aurora kinase inhibitors, but it also shows a vulnerability that could be exploited in future treatments. "I hope to find novel molecular targets for the selective killing of aneuploid cancer cells," says Noguchi.
Reference
- Noguchi, K., Hongama, K., Hariki, S., Nonomiya, Y., Katayama, K. & Sugimoto, Y. Functional effects of AKT3 on aurora kinase inhibitor-induced aneuploidy. Journal of Biological Chemistry 292, 1910-1924 (2017). | article