Adhesion of pathogenic bacteria to the intestinal wall activates an immune response
Published online 17 March 2016
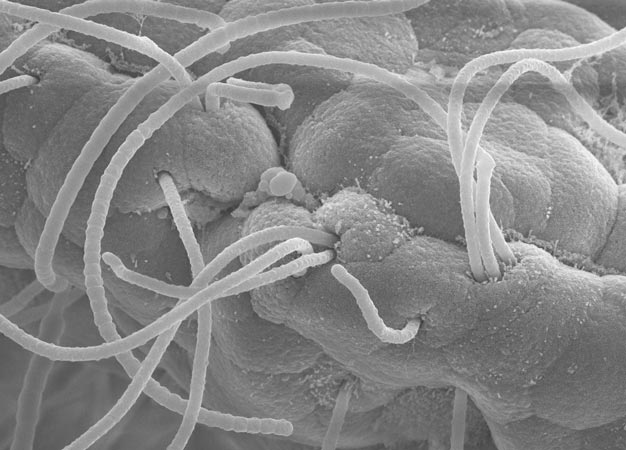
Electron micrograph of sticky segmented filamentous bacteria (long strands) attached to epithelial cells.
© 2016 Kiminori Toyooka and Seiko Narushima, RIKEN
Intestinal immune cells are spurred to action by the attachment of pathogenic bacteria to the gut lining, according to research from Keio University1. Their discovery highlights the importance of physical interaction in triggering the immune response, and could improve treatments to balance the overproduction or underproduction of these immune cells in various diseases.
Pathogenic bacteria invade the intestinal wall by adhering to the epithelium and disrupting the epithelial barrier, explains Kenya Honda, who led the study. "That's pathogenic bacteria's pattern: adhesion, disruption, and invasion."
Immune cells which are abundant in the small intestine, known as Th17 cells, block this process by stimulating epithelial cells to produce anti-microbial peptides and strengthen the tight junctions between them, which maintain the epithelial barrier.
In rodents, resident gut microbes known as segmented filamentous bacteria are potent inducers of Th17 activity. Receptors on the Th17 cells are known to recognize the foreign antigens, but Honda suspected that adhesion might also play an important role in triggering the immune response. "Segmented filamentous bacteria are very sticky bacteria," he says (see image).
To test the importance of physical contact, however, Honda's team first had to overcome the challenges of culturing the bacteria and developing non-adhesive mutants.
The breakthrough came with an experiment by Yoshinori Umesaki at the Yakult Central Institute. Umesaki introduced segmented filamentous bacteria from rats into microorganism-free mice, and vice versa. The reciprocal colonization experiments showed that adhesion is host-specific -- the foreign bacteria couldn't adhere to the intestinal lining of their new hosts -- and that Th17 cells weren't induced in the absence of bacterial adhesion.
To study the process in humans, the researchers had to identify similar Th17-inducing bacteria colonizing the human intestine. They isolated 20 strains present in fecal samples from ulcerative colitis patients that were not present in healthy patients. When these strains were introduced to microorganism-free mice, they adhered to the lining of the colon, where they activated Th17 cells.
Diseases like ulcerative colitis and Crohn's disease have been linked to an imbalance in the gut microbiome, so correcting the imbalance could cure the disease, or at least alleviate symptoms.
Eventually, Honda's team aims to use microbiome engineering as a way to treat patients with an overactive immune response, such as in autoimmune disorders, or a weak immune response. "For example, HIV-infected patients have fewer Th17 cells in their gut," explains Honda. "If we could identify a nice set of Th17-inducing bacteria, those could be applied to treat HIV or other diseases."
Reference
- Atarashi, K. et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163, 367-380 (2015). | article