Water crystals can purify natural gas by selectively trapping carbon dioxide molecules
Published online 21 January 2016
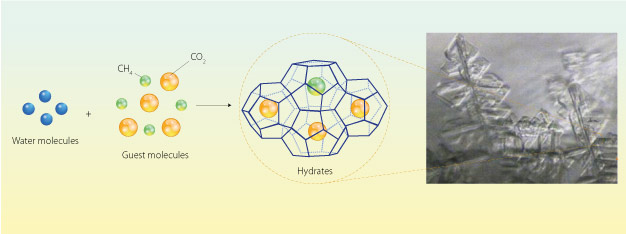
Hydrates, compounds that trap gas molecules inside cages of ice, have diverse applications from carbon capture to consumer goods.
© 2016 Ryo Ohmura, Keio University
Unconventional sources of natural gas, such as so-called 'flammable ice' reserves located in the Sea of Japan, may soon find wider use thanks to carbon capture technology developed by Keio researchers1.
Generating electricity from natural gas instead of coal or oil is a promising way to reduce greenhouse gas emissions. This energy, composed primarily of methane (CH4) and other light hydrocarbons, burns cleaner than other fossil fuels because it is relatively free of impurities like carbon dioxide (CO2) and hydrogen sulfide. However, rising demand means that lower-quality natural gas supplies, with trickier purification requirements, are being targeted for extraction.
Chemical absorption and membrane separation are typical techniques used to remove CO2 impurities from natural gas. But these treatments suffer from disadvantages such as the use of corrosive liquids and the need to constantly clean clogged filters. Ryo Ohmura and colleagues from Keio University's Department of Mechanical Engineering investigated a way to avoid such complications by using simple water molecules with surprising gas-trapping abilities.
Clathrate hydrates are ice-like complexes that form naturally on ocean seabeds, where temperatures plummet and pressures are enormous. These conditions cause water molecules to hydrogen-bond into cages and trap gases such as CH4 or CO2 at densities tens to hundreds of times greater than possible through typical compression techniques (see image).
The hydrates are also selective, explains Ohmura, and only stabilize when the gas compound fits suitably inside the aqueous cage: molecules too big or too small cause the complex to collapse. Since CO2 molecules are larger than CH4 molecules, the hydrates can be used to purify natural gas by selectively encapsulating and removing unwanted CO2.
The team tested the carbon-capturing performance of hydrates under conditions that model the continuous operations of natural gas refineries. Using a small-scale reactor, they passed a CO2-CH4 mixture through a hydrate slurry and analyzed compositions for over 35 hours. Eventually, steady-state conditions ― with significantly reduced CO2 concentrations ― were reached.
The researchers then compared the experimental data to computational algorithms designed to uncover the thermodynamic and kinetic factors behind hydrate purification. "With more accurate models, we can identify ways to improve the hydrate formation, and understand long-term CO2 capture more precisely," says Ohmura.
This technology has other, intriguing applications that could even change what we eat for dessert. Ohmura and his team are developing hydrates embedded with CO2 that can carbonate frozen food ― a potential way to inject champagne-like effervescence into ice creams and jellies.
Reference
- Tomita, S., Akatsu, S. & Ohmura, R. Experiments and thermodynamic simulations for continuous separation of CO2 from CH4 + CO2 gas mixture utilizing hydrate formation. Applied Energy 146, 104-110 (2015). | article