Reprogrammed stem cells show how a druggable hormone helps drive the problems associated with hypertrophic cardiomyopathy
Published online 6 March 2015
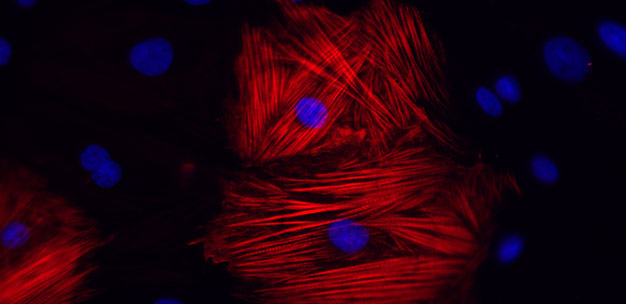
Cardiomyocytes derived from the reprogrammed cells of a patient with hypertrophic cardiomyopathy show augmented disarray in the presence of the hormone endothelin-1.
© 2015 Shinsuke Yuasa, Keio University
An in vitro model of the hereditary heart disorder hypertrophic cardiomyopathy has revealed how genetic backgrounds interact with environmental factors to cause the hallmark phenotypes of the disease1.
In hypertrophic cardiomyopathy, heart muscle cells increase in size, leading to thickening that makes it hard for blood to flow. The alignment of the cells also becomes disordered and irregular, causing changes in electrical signals that can trigger life-threatening abnormal heart rhythms. The condition is the leading cause of sudden unexpected death in young athletes and can cause debilitating cardiac problems in any age group.
To better understand the root cause of the disease, a team led by Keiichi Fukuda and Shinsuke Yuasa from Keio University School of Medicine took skin cells or blood cells from three unrelated patients with hypertrophic cardiomyopathy and three healthy controls. They introduced a virus expressing four pluripotency genes ― the so-called 'Yamanaka factors', named after the Japanese Nobel laureate Shinya Yamanaka ― and created embryonic-like 'induced pluripotent stem cells'. The researchers then coaxed these reprogrammed stem cells to form heart muscle cells called cardiomyocytes.
Under normal culture conditions, the morphological features and cellular behaviors of the cardiomyocytes from the healthy and diseased subjects appeared largely the same. However, when the researchers exposed the cells to endothelin-1, a hormone known to trigger the constriction of blood vessels, they observed large differences in the architecture of rod-like muscle fibrils (see image).
High-speed imaging studies further revealed that the heart muscle cells from patients with the condition beat out of sync when subjected to treatment with endothelin-1. This was true whether the cardiomyocytes came from stem cells derived from someone with a mutation in MYBPC3, a gene that is often dysfunctional in people with hypertrophic cardiomyopathy, or from patients with the disease but no known causative mutation.
The researchers confirmed their findings in a mouse model. Using cardiomyocytes derived from newborn mice with a heterozygous mutation in the mouse version of the MYBPC3 gene, they showed that endothelin-1 induced similar problems in muscle fibrils to those seen in the human cells.
Several drugs that block endothelin receptors are already on the market to treat another heart condition called pulmonary arterial hypertension. The findings from Fukuda, Yuasa and their colleagues suggest that these same agents might help people with hypertrophic cardiomyopathy as well. "We are planning a clinical study to know if an endothelin-1 blocker could be a standard therapy," says Yuasa.
Reference
1.Tanaka, A. et al. Endothelin-1 induces myofibrillar disarray and contractile vector variability in hypertrophic cardiomyopathy-induced pluripotent stem cell-derived cardiomyocytes. Journal of the American Heart Association 3, e001263 (2014). | article