A microfluidic instrument that can rapidly screen millions of electron-transferring enzymes may uncover the next biofuel device
Published online 6 March 2015
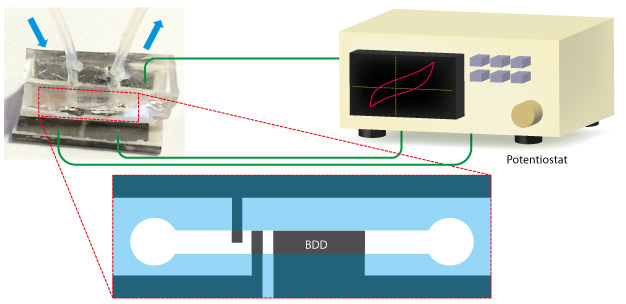
Microchip devices containing boron-doped diamond (BDD) microelectrodes can screen enzymes for catalytic activity with remarkably high throughput, measured using a potentiostat.
Reproduced, with permission, from Ref. 1 © 2014 American Chemical Society
The power output and stability of biofuel cells that generate cheap electricity with catalytic enzymes can now be improved thanks to an analytic instrument developed at Keio University1. Nobuhide Doi from the Department of Biosciences and Informatics and colleagues have fabricated a device that screens libraries of potential enzyme catalysts hundreds to thousands of times faster than conventional methods using micro-sized electrodes and microfluidic sample handling.
NAD(P)-dependent oxidoreductases are enzymes that catalyze electron transfer between a broad range of biomolecules. This versatility has led researchers to investigate if the enzyme could replace heavy metal catalysts in electricity-generating fuel cells. So far, however, the oxidoreductases tested in biofuel cells have shown insufficient activity and stability for practical applications.
One way to improve the properties of existing oxidoreductases is with a process called directed enzyme evolution that mimics natural evolution to build up massive libraries of enzymes bearing slight mutations in their structures. Then, screening and selection experiments test the mutated enzymes' catalytic properties to help guide the evolution process. Current screening techniques based on light adsorption can assess thousands of clones at once, but higher-throughput methods are needed to advance fuel cell development.
Doi and his team explored whether the catalytic ability of NAD(P)-dependent oxidoreductases could be detected by measuring their electrochemistry ― a significantly faster screening method that requires only tiny sample volumes. Achieving this goal required special 'boron-doped diamond (BDD) microelectrodes' developed by Yasuaki Einaga at Keio University. These robust, needle-shaped devices can survey a wide range of electrochemical conditions while having very low background current, making them ideal for ultra-sensitive detection of enzyme activity.
The team constructed a tube-shaped microfluidic device that continuously flows small volumes of enzymes past a BDD microelectrode detector (see image). After optimizing for pH and anti-protein aggregation effects, they tested the activity of two important catalytic enzymes: glucose-6-phosphate dehydrogenase and alcohol dehydrogenase. Remarkably, the new instrument needed only a millisecond to detect part-per-trillion concentration levels of the dehydrogenases, and required mere nanoliters of sample volumes. "The cost and time can be saved significantly compared with conventional methods," says Doi.
The speed and accuracy of the microfluidic-BDD microelectrode device implies it could handle much larger screening libraries than current techniques ― potentially up to one million enzyme clones. Doi is confident this approach can narrow the search for 'super enzymes' appropriate for biofuel cells, likening it to a gambler improving his odds. "The more lottery tickets you buy, the higher your chance of winning," he adds.
Reference
- Oyobiki, R. et al. Toward high-throughput screening of NAD(P)-dependent oxidoreductases using boron-doped diamond microelectrodes and microfluidic devices. Analytical Chemistry 86, 9570-9575 (2014). | article