A simple route to immobilizing nanoclusters opens up new possibilities in materials chemistry
Published online 6 March 2015
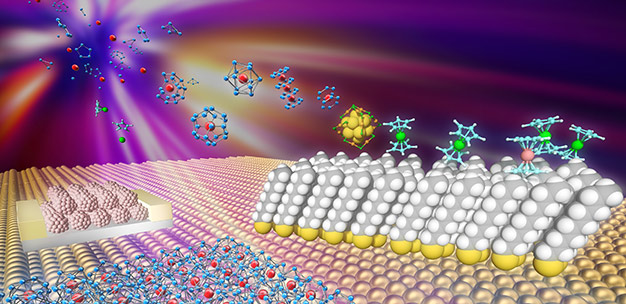
Nanoclusters of caged structures (blue and red) and sandwich structures (blue and green) synthesized in the gas phase can be deposited onto designer surfaces such as buckyball-functionalized conductive surfaces (center left) and alkanethiolate self-assembled monolayers (center right) as well as naked metal surfaces (bottom), with a range of possible applications in materials chemistry.
© 2015 Atsushi Nakajima, Keio University
Researchers have developed a technique for assembling novel nanomaterials from clusters of gaseous ions, with potential implications for solar energy, energy storage and high-performance catalysis1.
Small clusters of silicon cages holding single metal atoms or ions have long been identified as potential building blocks for nanomaterials. Before they can be used, however, these gaseous nanoclusters need to be immobilized on a solid surface. Previous attempts have resulted in undesirable changes to the shape and electronic charge of the clusters.
Atsushi Nakajima from the Keio Institute of Pure and Applied Sciences and colleagues at Keio University have immobilized tantalum cations trapped inside cages comprised of 16 silicon atoms (Ta@Si16+) onto a conductive surface while retaining their vital original properties.
The team selected a conductive surface functionalized with buckyballs (C60) that are able to act as electron acceptors, theorizing that the Ta@Si16+ cations and buckyballs would form charge-transfer complexes (Ta@Si16+-C60−). "The immobilization process consists of two steps," explains Nakajima. First the Ta@Si16+ is neutralized, and then it transfers an electron to the buckyball and becomes cationic again. This transfer of an electron between each cluster and the buckyball it lands on is "a crucial factor in maintaining its symmetric cage shape and cationic state," he explains.
The researchers experimentally verified their theory by vaporizing tantalum and silicon atoms in a vacuum chamber to make the Ta@Si16+ cations. They then used mass spectrometry to identify the desired ions from the range of differently sized nanoclusters that had formed. The selected ions were fired onto the buckyball-functionalized graphite surface at a tightly controlled rate to ensure a gentle landing.
The researchers used scanning tunneling microscopy and scanning tunneling spectroscopy to study the structure of the resulting nanomaterial. A charge-transfer complex had indeed formed, creating a monolayer of the Ta@Si16+ cations on top of the anionic buckyballs attached to the conductive surface.
"This fabrication approach can be used to make a range of nanomaterials comprised of both different nanoclusters and different surfaces," explains Nakajima. Such nanomaterials made of monolayers of cations next to monolayers of anions could act as electrical junctions in a range of devices such as solar cells and supercapacitors. The technique could also lead to the design of high-performance catalysts. It has been predicted that the cationic state of supported nanoclusters is a key factor for controlling their catalytic reactivity, says Nakajima. "A novel avenue for developing nanocluster-based material science and technology has opened to us."
Reference
1. Nakaya, M., Iwasa, T., Tsunoyama, H., Eguchi, T. & Nakajima, A. Formation of a superatom monolayer using gas-phase-synthesized Ta@Si16 nanocluster ions. Nanoscale 6, 14702 (2014). | article