A reusable reagent offers a greener way to make carbohydrate-containing molecules
Published online 6 March 2015
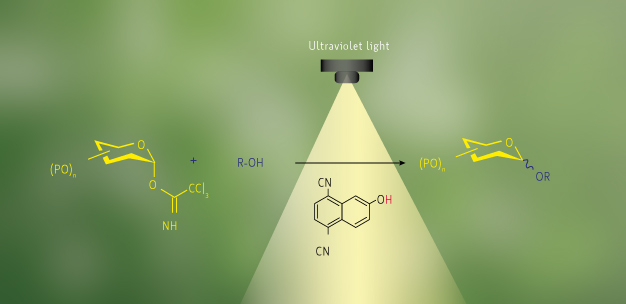
The environmentally benign glycosylation reaction for the coupling of carbohydrates (yellow) and alcohols (blue) using organic acids (black).
© 2015 Kazunobu Toshima, Keio University
The synthesis of organic molecules using methods that result in minimal waste and take advantage of renewable energy sources has been a long-term goal for chemists. Researchers at Keio University have shown that certain organic acids can facilitate the coupling reaction between carbohydrates and various alcohols, and then be recovered and reused without any loss of reaction efficiency1. The glycosylation reaction, as well as benefiting from the recyclability of the acidic reagent, is triggered by light ― a clean energy source.
"Our glycosylation reaction is highly environmentally benign compared with conventional methods," says study author Kazunobu Toshima. "Moreover, it is the first example of a synthetic organic reaction employing reusable organic acids, which have been activated by photo-irradiation."
Glycosylation involves attaching a carbohydrate to other sugars or organic molecules to yield oligosaccharides or glycosides. Carbohydrate-containing molecules such as glycoproteins and glycolipids are vital participants in many biological processes. Glycosylation reactions are also common steps in the synthesis of bioactive and functional products, ranging from antibiotics to biodegradable surfactants. Given their widespread application, novel and environmentally benign routes to glycosylated products are highly sought after.
With this in mind, Toshima and colleagues identified several organic acids that can act as photo-activated reagents for glycosylation (see image). The organic acids comprise aromatic molecules with hydroxyl groups attached directly to the aromatic system ― more specifically, phenol or naphthol derivatives. Photo-irradiation with ultraviolet light increases the acidity of the organic acids, which causes a trichloroacetimidate group to leave the glycosyl donor and creates an intermediate suitable for reaction with various alcohols. The electron-donating capability of the organic acids was not sufficient to react with this intermediate, so no unwanted coupling with the hydroxyl group of the acids occurred.
The researchers then removed the ultraviolet light source to neutralize the reaction mixture and applied heat at a relatively low temperature to isolate the glycosylated product from the recyclable organic solvent, ether. The organic acids could be recovered in greater than 90 per cent yield, after purification of the reaction mixture using column chromatography, and reused with no loss of efficiency.
Toshima sees few hurdles in scaling up the reaction. "A large-scale reaction would only need a more powerful light source and a larger reaction vessel," he says.
"We envisage the development of different types of synthetic organic reactions using these light-activated organic acids, for example, carbon-carbon bond formation reactions, such as Mannich or Strecker reactions," he notes.
Reference
- Iwata, R., Uda, K., Takahashi, D. & Toshima, K. Photo-induced glycosylation using reusable organophotoacids. Chemical Communications 50, 10695-10698 (2014). | article