Advances in iPS cell-based therapy for treatment of hereditary hearing loss
Published online 28 September 2018
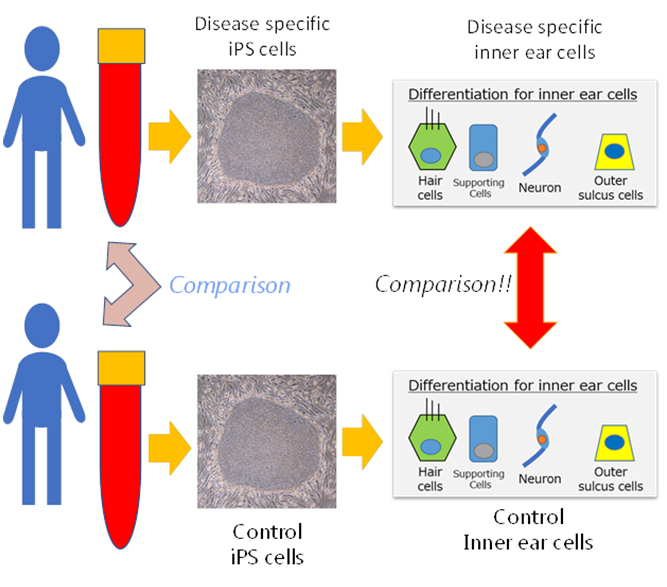
Application of iPS cells in studying hereditary hearing loss
© Department of Otorhinolaryngology, Head and Neck Surgery, Keio University School of Medicine
Masato Fujioka, Makoto Hosoya, and their colleagues at the Department of Otorhinolaryngology, Head and Neck Surgery, Keio University School of Medicine, are searching for methods to treat genetic hearing loss ― an extremely challenging area of medicine with no potentially useful strategies to cure such ailments at present.
Fujioka and Hosoya are investigating the possibility of using regenerative medicine, specifically iPS cells, to model human inner ear cells. "Ideally we would like to obtain samples of inner ear tissues by direct biopsy," says Fujioka. "But in reality, this is not possible because the inner ear is filled with lymph inside the bone that makes it inaccessible to collect cells for examination and observation to monitor progression of hearing loss. So, we devised a new approach based on first producing iPS cells from patients and then inducing inner ear cells from these iPS cells. Our early results are very promising and we expect them to lead to in vitro procedures for pathophysiological analysis and drug screening."
The Keio researchers used patient-derived iPS cells to discover the cause of one of the most common forms of hereditary hearing loss, Pendred syndrome1. Pendred syndrome causes progressive deafness and no effective therapies are available. In genetically modified mouse models, however, the disease does not lead to progressive deafness as is observed in humans.
The iPS cells were prepared from the blood of patients and used to induce cells of the inner ear. The mechanisms causing deafness were then studied. The results showed that the abnormal protein known as pendrin only accumulated in the patient's cells of the inner ear, and notably, that this protein was made of aggregates similar to those of neurodegenerative diseases such as Alzheimer's disease.
Further analysis showed the inner ear cells to be vulnerable to cellular stress, and that hearing loss gradually progresses due to the death of a particular type of inner ear cell--a finding proposed as "inner ear degeneration."
The researchers also searched for therapeutic drugs to prevent cell death and discovered for the first time the possible therapeutic effects of rapamycin, which is already used as an immunosuppressant. This research utilizing patient iPS cells yielded unexpected findings that imply phenomena observed in Alzheimer's disease are also occurring in the inner ear. This pioneering research opens up the possibility of a major breakthrough in the treatment of deafness, including senile deafness.
The future role of artificial intelligence and the Internet of Things
"I was interested in regenerative medicine during my high school days so I wanted to study at the Keio University School of Medicine," says Hosoya. "I was really impressed by the research of Keio's Professor Okano, Physiology, and Professor Fujioka, Otolaryngology, so I decided to start conducting research on inner ear regeneration. Now, as medical doctors we have to expand our horizons and learn about artificial intelligence (AI) as well."
The Keio team stresses the increasing inroads AI is making in medicine, in particular radiology, pathology, dermatology, and diagnosis. "I think that similar waves of AI will reach otorhinolaryngological diseases," says Fujioka. "I think that the biggest challenge for advances in inner ear medicine is collecting physiological data. The conditions of patients can change rapidly, such as in Ménière's disease, and physicians must be aware of them. So, I would like to develop secure procedures to collect accurate, time-dependent physiological data of the functions of the inner ear."
As an example, Fujioka describes the treatment of Pendred syndrome, a disorder characterized by hearing loss and repetitive dizziness where the conditions of patients can vary significantly with time. Frequent hearing tests are required to monitor the effects of treatment. This is a perfect example of lending equipment to patients to collect biological information with Internet of Things (IoT) technology that could be monitored in real time by the patient's physician.
"We are planning IoT trials with 16 patients for 10 months," explains Fujioka. "So, three physiological examinations per person per day will produce about 300 days of data. We will monitor the effects of drug administration on the patient's symptoms." Fujioka adds that by monitoring changes in the inner ear function in the same patient and using machine learning algorithms, it may be possible to predict vertigo attacks before they occur.
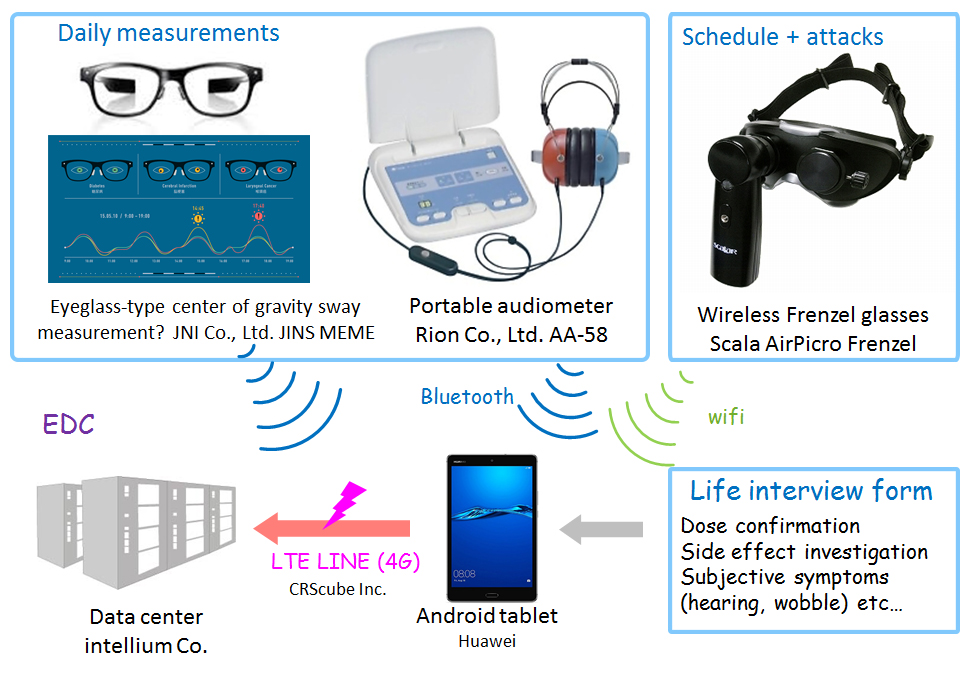
IoT-based monitoring of patients with ear ailments
© Department of Otorhinolaryngology, Head and Neck Surgery, Keio University School of Medicine
Trials on 16 people will yield 4,800 sets of data, and 14,400 measurement results--a significant amount of "big-data." The point is that all 16 patients being monitored are limited to the mutations of the same gene, and "several different mutations in one single gene will be included in our trials," says Fujioka. There are expected to be individual differences, but it is a homogeneous population compared to Ménière's disease and senile deafness. It is expected to be an ideal group to create prototypes of analysis algorithms.
"I hope that this IoT clinical trial system can become a prototype for future clinical trials for inner ear disorders, as well as a health management tool for a wide range of users handling easy to use equipment that is not necessarily medical devices used in hospitals."
About the researchers

Masato Fujioka― Assistant Professor (left) and Makoto Hosoya ― Research Associate
Department of Otorhinolaryngology, Head and Neck Surgery, Keio University School of Medicine.
Masato Fujioka received his M.D. and Ph.D. in otorhinolaryngology in 2002 and 2006, respectively, from Keio University. From 2006 to 2009 he was a senior research fellow at the Department of Otology and Laryngology, Harvard Medical School and worked at the Eaton-Peabody Laboratory, Massachusetts Eye and Ear Infirmary. From 2009 to 2015 he was an instructor at the Department of Otorhinolaryngology Head and Neck Surgery, Keio University School of Medicine. Between 2011 and 2013 he worked at Keiyu Hospital, Yokohama, as an attending doctor in ENT. He was appointed assistant professor at Keio University in 2016.
His research covers from basic science to translational research in the field of auditory and/or vestibular systems. Specific research topics include autoimmunity, inflammation, regeneration and stem cell biology. He is the principal investigator of a clinical trial of iPSC-based drug development for rare intractable genetic hearing loss Pendred syndrome/DFNB4.
- Awards -
10th. International Society for Stem Cell Research
Young Investigator Award, Keio University School of Medicine Alumni Association
14th. Harold F. Schuknecht Travel Award
Makoto Hosoya received his M.D. and Ph.D. in 2010 and 2017, respectively, From 2017 he belongs to the Department of Otorhinolaryngology, Head and Neck surgery, Keio University School of Medicine.
His research interests cover stem cell technology, hearing regeneration and mechanisms of hereditary hearing loss. Specific aims are to clarify mechanisms underlying hearing regeneration and species differences underlying the genetic hearing loss.
References
-
Makoto Hosoya, Masato Fujioka, Takefumi Sone, Satoshi Okamoto, Wado Akamatsu, Hideki Ukai, Hiroki R. Ueda, Kaoru Ogawa, Tatsuo Matsunaga, and Hideyuki Okano, "Cochlear Cell Modeling Using Disease-Specific iPSCs Unveils a Degenerative Phenotype and Suggests Treatments for Congenital Progressive Hearing Loss", Cell Reports, 18, P68-81, January 03, 2017. | article